Abstract
In the present study, we have analyzed the pattern of cytokines expressed by two independent dendritic cell (DC) subpopulations generated in vitro from human cord blood CD34+ progenitors cultured with granulocyte-macrophage CSF and TNF-α. Molecularly, we confirmed the phenotypic differences discriminating the two subsets: E-cadherin mRNA was only detected in CD1a+-derived DC, whereas CD68 and factor XIIIa mRNAs were observed exclusively in CD14+-derived DC. Semiquantitative reverse-transcriptase PCR analysis revealed that both DC subpopulations spontaneously expressed IL-1α, IL-1β, IL-6, IL-7, IL-12 (p35 and p40), IL-15, IL-18, TNF-α, TGF-β, macrophage CSF, and granulocyte-macrophage CSF, but not IL-2, IL-3, IL-4, IL-5, IL-9, and IFN-γ transcripts. Both subpopulations were shown to secrete IL-12 after CD40 triggering. Interestingly, only the CD14+-derived DC secreted IL-10 after CD40 activation, strengthening the notion that the two DC subpopulations indeed represent two independent pathways of DC development. Furthermore, both DC subpopulations expressed IL-13 mRNA and protein following activation with PMA-ionomycin, but not with CD40 ligand, in contrast to IL-12 and IL-10, revealing the existence of different pathways for DC activation. Finally, we confirmed the expression of IL-7, IL-10, and IL-13 mRNA by CD4+CD11c+CD3− DC isolated ex vivo from tonsillar germinal centers. Thus, CD14+-derived DC expressing IL-10 and factor XIIIa seemed more closely related to germinal center dendritic cellsGCDC than to Langerhans cells.
Dendritic cells (DC),4 the most potent APC found at trace levels in lymphoid and nonlymphoid tissues, are required for the priming of naive T lymphocytes (1). Immature DC, such as Langerhans cells in the epidermis, have the ability to capture Ags, and to become circulating veiled cells. These cells transport the Ags via blood or lymph vessels to lymphoid organs, where they mature into interdigitating DC (2, 3, 4, 5, 6). At this stage of terminal differentiation, they efficiently present processed Ags to naive T cells and induce an Ag-specific primary T cell response (7, 8). Several cytokines strongly influence the commitment of naive T cells activated through their TCR toward distinct effector functions (9).
Difficulty to isolate DC ex vivo has limited our current knowledge regarding the cytokines produced by different DC subsets. After exposure to contact allergens in vivo, Langerhans cells up-regulate IL-1β mRNA expression, suggesting an important role for this cytokine in the initiation of primary immune responses in the skin (10). IL-12 production by DC, which has been documented by several authors (11, 12, 13, 14), favors the differentiation of Th0 cells into Th1 cells (11, 15). Scheicher et al. (16) have reported that uptake of particle-adsorbed Ag by DC up-regulates the transcription of both IL-1α and IL-12 (p35 and p40). Finally, blood-derived DC were shown to express mRNA for numerous cytokines (17, 18) and to secrete functional IL-15 (19).
Study of DC has recently been facilitated by the development of in vitro culture systems, allowing the generation of large number of highly pure DC (20, 21, 22, 23). In this context, human cord blood CD34+ hemopoietic progenitors cultured in presence of GM-CSF and TNF-α were shown recently to differentiate along two independent DC pathways (24, 25). Thus, CD1a+-derived DC, related to epidermal Langerhans cells, are characterized by the expression of Birbeck granules, Langerhans-associated granule Ag, and E-cadherin (26, 27). In contrast, CD14+-derived DC, expressing CD68 and factor XIIIa (two dermal DC markers), are more closely related to interstitial DC and/or peripheral blood DC. Whereas both DC subpopulations are equally potent in stimulating naive T cell proliferation, CD14+-derived DC are more efficient in Ag uptake and have the unique capacity to induce naive B cells to differentiate into IgM-secreting cells (28). To understand the functional differences between these two DC subpopulations generated in vitro, their pattern of cytokine expression was analyzed by semiquantitative RT-PCR. Both cell types express a large array of cytokine mRNAs, including IL-7, and secrete IL-13, but not IL-4, upon PMA-ionomycin activation. Of particular interest, the production of IL-10 mRNA and protein is restricted exclusively to CD14+-derived DC. This represents a major difference between the two DC subsets, given the strong effect of IL-10 on T cell priming (29, 30). Different activation signals up-regulate different cytokines: CD40 engagement induces IL-12 and IL-10 secretion, while PMA-ionomycin activation turns on IL-13 production. Finally, IL-7, IL-10, and IL-13 were also expressed by GCDC isolated ex vivo (31), suggesting close relationship with CD14+-derived DC. Taken together, these results indicate that the outcome of a primary immune response will depend on which subset of DC is involved, as well as on the activation signal delivered to the DC during the early phase of the response.
Materials and Methods
Hemopoietic factors, cells, and cell lines
rhGM-CSF (sp. act., 2 × 106 U/mg; Schering-Plough Research Institute, Kenilworth, NJ) was used at a saturating concentration of 100 ng/ml (200 U/ml). rhTNF-α (sp. act., 2 × 107 U/mg; Genzyme Corp., Boston, MA) was used at an optimal concentration of 2.5 ng/ml (50 U/ml). rhSCF (sp. act., 4 × 105 U/mg; R&D, Abington, U.K.) was used at an optimal concentration of 25 ng/ml.
PBMC were obtained from healthy donors after Ficoll-Hypaque gradient centrifugation (d = 1.077; Eurobio, Paris, France). Cells were activated by PMA-ionomycin for 6 h (PMA, 1 ng/ml; Sigma Chemical Co., St. Louis, MO) (ionomycin, 1 μg/ml; Calbiochem, La Jolla, CA). MT9 is a CD4 T cell clone obtained in the laboratory; the cells were stimulated by PMA-ionomycin for 6 h. Murine fibroblasts transfected with human CD40 ligand (CD40L L cells) were produced in the laboratory (32). All cell types were cultured in RPMI 1640 (Life Technologies, Gaithersburg, MD) supplemented with 10% (v/v) heat-inactivated FBS (Flow Laboratories, Irvine, U.K.), 10 mM HEPES, 2 mM l-glutamine, 5 × 10−5 M 2-ME, penicillin (100 U/ml), and streptomycin (100 μg/ml) (hereafter referred to as complete medium).
Purification of cord blood CD34+ hemopoietic progenitor cells
Umbilical cord blood samples were obtained according to institutional guidelines. Cells bearing CD34 Ag were isolated from mononuclear fractions through positive selection, using anti-CD34 mAb (Immu-133.3; Immunotech, Marseille, France) and goat anti-mouse IgG-coated microbeads (Miltenyi Biotec GmbH, Bergish Gladbach, Germany). Isolation of CD34+ progenitors was achieved using Minimacs separation columns (Miltenyi Biotec GmbH), as described (24). In all experiments, the isolated cells were 80 to 99% CD34+, as judged by staining with anti-CD34 mAb. After purification, CD34+ cells were cryopreserved in 10% DMSO.
Generation of DC from CD34+ cell culture
Cultures were established in the presence of SCF, GM-CSF, and TNF-α, as described, in complete medium (20, 24). After thawing, CD34+ cells were seeded for expansion in 25- to 75-cm2 flasks (Corning, New York, NY) at 1 to 2 × 104 cells/cm2. Optimal conditions were maintained by splitting these cultures at day 4 with medium containing fresh GM-CSF and TNF-α (cell concentration, 1–3 × 104 cells/cm2). For most experiments, cells were routinely collected after 5 to 6 days of culture for FACS sorting (24). Culture medium was supplemented with 5% AB+ pooled human serum at initiation of the cultures, and by day 5 to 6, human serum was washed away. Unsorted cells were cultured in presence of GM-CSF and TNF-α until day 12.
CD1a+ and CD14+ cell FACS sorting
After 5 to 6 days of culture in presence of SCF, GM-CSF, and TNF-α, cells were collected and labeled with FITC-conjugated OKT6 (CD1a; Ortho Diagnostic Systems, Raritan, NJ) and PE-conjugated Leu-M3 (CD14; Becton Dickinson, Mountain View, CA). Cells were separated according to CD1a and CD14 expression into CD14+CD1a− and CD14−CD1a+, using a FACStarPlus (Becton Dickinson) (laser setting: power, 250 mW; excitation wavelength, 488 nm). To obtain highly purified populations of CD1a+- and CD14+-derived cells, DC precursors were sorted twice. Reanalysis of the sorted populations showed a purity higher than 99% (T cells could never be detected, even by PCR). Sorted cells were seeded in the presence of GM-CSF and TNF-α (0.5–1 × 105 cells/cm2) for 6 to 7 additional days, a last medium change being performed at day 10. At day 12 of the culture, cells were divided into three fractions: one was untreated, whereas the two others were stimulated either by PMA-ionomycin for 6 h, or by irradiated CD40L L cells for 24 h (1 CD40L L cell for 5 DC).
Purification of GCDC from tonsils
Germinal center dendritic cells (GCDC) were prepared as previously described (31). In brief, tonsils obtained from children undergoing tonsillectomy were finely minced and digested with collagenase IV and DNase. The collected cells were centrifuged through Ficoll-Hypaque for 15 min at 500 rpm, then for 30 min at 2000 rpm. CD3+ T cells, CD19+ B cells, and CD14+ monocytes were removed from the resulting low density cells by magnetic beads (anti-mouse Ig-coated Dynabeads; Dynal, Oslo, Norway). Anti-CD3 (OKT3), anti-CD19 (4G7), and anti-CD14 (MOP9) mAb were purified from ascites. A second depletion was performed with anti-NKH1 (Coulter Corp., Hialeah, FL), anti-glycophorin A (Immunotech), and anti-CD20 (purified from ascites). The remaining cells were stained with the following mAbs: anti-CD1a FITC (Ortho Diagnostic Systems); anti-CD14 FITC, anti-CD57 FITC, anti-CD16 FITC, anti-CD7 FITC, anti-CD20 FITC, and anti-CD3 FITC (Becton Dickinson); and anti-CD4 PE-Cy5 (Immunotech) and anti-CD11c PE (Becton Dickinson). CD4+CD11c+CD3−CD20−CD1a− GCDC were isolated by cell sorting using a FACStarPlus (Becton Dickinson). A quantity amounting to 2 to 5 × 109 tonsil cell suspension was required to purify 2 × 105 to 2 × 106 GCDC with a purity higher than 97%.
GCDC were stimulated by either PMA-ionomycin for 3 h or an anti-CD40 mAb (10 μg/ml of G28-5 mAb kindly provided by Ed. Clark, University of Washington, Seattle, WA).
RNA extraction
Total RNA was extracted from the cells following established procedures (33). Briefly, the cells (1–10.106) were washed twice with PBS; lysed in 4 M guanidine isothiocyanate (100 μl/1.106 cells), 25 mM sodium acetate (pH = 7), 0.5% N-lauroylsarcosine, and 100 mM 2-ME; vortexed; and frozen at −20°C until used. Cell lysates were extracted once with acidic phenol (pH 4.8) and once with chloroform-isoamyl alcohol (24:1). Precipitation was performed with 2 vol of absolute ethanol, and lysates were centrifuged at 13,000 rpm for 30 min. The pellet was washed with 70% ethanol, and redissolved in diethylpyrocarbonate 0.1%, water.
Reverse transcriptase-PCR
Total RNA obtained following the procedure described above was reverse transcribed using a random hexamer pN6 and Superscript RNase-H reverse transcriptase (Life Technologies, Bethesda, MD). PCR was conducted in a 100 μl vol using 1 μl cDNA, 10 μl 10× PCR reaction buffer (Perkin-Elmer Corp., Norwalk, CT), 2.5 U Taq polymerase (Gene Amp PCR reagents kit; Perkin-Elmer), 200 mM dNTPs, and 500 nM of the 5′ and 3′ amplification primers. The PCR was performed in a DNA thermal cycler (Perkin-Elmer) for 40 cycles (1-min denaturation at 94°C, 2-min annealing at 60°C, and 3-min elongation at 72°C). β actin mRNA amplification was performed on the cDNA as positive control of reaction efficiency. To evaluate mRNA expression semiquantitatively, in addition to the PCR product from the 40 cycles, 15 μl of the PCR product from the 28 cycles and the 35 cycles was run simultaneously on 1% agarose gels and transferred to nylon membranes. Negative controls were performed by omitting cDNA. As positive controls, RNA from cells known to abundantly express the respective mRNA was used: PMA-ionomycin-stimulated normal human PBMC or elutriated monocytes from PBMC.
Oligonucleotide primers and the expected sizes of PCR products from cytokines are listed in Table I. Sense and antisense primers for β actin, IL-2, IL-3, IL-5, and IL-12 p35 were obtained from Stratagene (La Jolla, CA).
Oligonucleotide primers and PCR product sizes for cytokine cDNAs
| mRNA . | Sense Primer . | Antisense Primer . | PCR Product (bp) . | Probe . |
|---|---|---|---|---|
| β-Actin | Stratagene | Stratagene | 661 | |
| IL-1α | 5’ATGGCCAAAGTTCCAGACATGTTT | 5’GTGACTGCCCAAGATGAAGACCAA | 600 | 5’TCCATCACTGATGATGACCTGGAGGCCATC |
| IL-1β | 5’ATGATGGCTTATTACAGTGGCAAT | 5’TTCACCATGCAATTTGTGTCTTCC | 777 | 5’ATGGAGCAACAAGTGGTGTTCTCCATG |
| IL-2 | Stratagene | Stratagene | 457 | 5’CCTCTGGAGGAAGTGCTAA |
| IL-3 | Stratagene | Stratagene | 449 | 5’CCATATCAAGGACGGTGACT |
| IL-4 | 5’CTGCTTCCCCCTCTGTTCTT | 5’CTGTGAAGGAAGCCAACCAG | 378 | 5’ACAGACATCTTTGCTGCCTC |
| IL-5 | Stratagene | Stratagene | 295 | 5’TTCAGGGAATAGGCACACTG |
| IL-6 | 5’AGTTGCCTTCTCCCTGG | 5’TGAGGGCTCTTCGGCAAAT | 621 | 5’CCAGCCTGCTGACGAAGCTGCAGGCACAG |
| IL-7 | 5’TTTTATTCCGTGCTGCTCGC | 5’GGTCAAAACGGATTAGGGCA | 430 | 5’TGGAATAAAATTTTGATGGGCACTAAAGAACACTGA |
| IL-8 | 5’ATGACTTCCAAGCTGGCCGTGGCTCTCTTG | 5’CTTACCTCACAGTGATGTTGTGAGGACATG | 886 | 5’GAGAAGTTTTTGAAGAGGGCTGAGAATTCA |
| IL-9 | 5’ATGCTTCTGGCCATGGTCCTTACCTCTGCCCTG | 5’GGGATGAGAGGCAAGATATGAAGATGAAATATT | 476 | 5’CAGTTGTCTCTGTTTGGGCA |
| IL-10 | 5’ATGCCCCAAGCTGAGAACCAAGACCCA | 5’TGGGATAGCTGACCCAGCCCCTT | 352 | 5’ACAATGAAGATACGAAACTGAGACATCAGG |
| IL-12 p40 | 5’GGATGCCCCTGGAGAAATGG | 5’AGGTGGAGGTCAGCTGGGAG | 655 | 5’TGCTGGTGGCTGACGACAAT |
| IL-12 p35 | Stratagene | Stratagene | 870 | 5’GCCCTGTGCCTTAGTAGTAT |
| IL-13 | 5’GCTCTTGCTTGCCTTGGTGG | 5’AAGCAACTGTTTCGCCACGG | 359 | 5’GCCATCTACAGGACCCAGAG |
| IL-15 | 5’CTCCCTAAAACAGAAGCCAAC | 5’GCAAAGAATGTGAGGAACTGG | 292 | 5’TGAAGTGCTTTCTCTTGGAG |
| IL-18 | 5’TTCGGGAAGAGGAAAGGAAC | 5’AAGGATACAAAAAGTGACAT | 480 | 5’GACTGATTCTGACTGTAGAG |
| TNFα | 5’GTTCCTCAGCCTCTTCTCCT | 5’ATCTATCTGGGAGGGGTCTT | 507 | 5’ACCCCGAGTGACAAGCCTGTAGCCCATGTT |
| TGFβ | 5’AAGCAGAGTACACACAGCATATATATGTTC | 5’ATTTGGAGCCTGGACACGCAGTACAGCAAG | 645 | 5’CTTCTCATGGACACCCCGCTGGAGAGGGCC |
| M-CSF | 5’GCAGGAGTATCACCGAGGAG | 5’CTCCCTCTTGCCTGGTGAG | 633 | 5’AGATAACACCCCCAATGCCA |
| GM-CSF | 5’AGCCCCAGCACGCAGCCCTGG | 5’CCCTTTGACTGCTGGGAGCCAGTCCAGGAG | 363 | 5’CGGGGCAGCCTCACCAAGCTCAAGGGCCC |
| IFNγ | 5’CTGTTACTGCCAGGACCCATATGTAAAAG | 5’CAAGGTCGAAGAGCATCCCAGTAATGGTTG | 448 | 5’GCGGATAATGGAACTCTTTTCTTAGGCAT |
| E-cadherin | 5’CCGATTCAAAGTGGGCACAG | 5’GCCATCGCTTACACCATCCT | 696 | 5’CCCCCTGTTGGTGTCTTTAT |
| Factor XIIIa | 5’TGTCAGAAACTTCCAGGACC | 5’CTGGACTGGAAGCGTTGACA | 954 | 5’GCAGATTGACTTCAGTCGT |
| mRNA . | Sense Primer . | Antisense Primer . | PCR Product (bp) . | Probe . |
|---|---|---|---|---|
| β-Actin | Stratagene | Stratagene | 661 | |
| IL-1α | 5’ATGGCCAAAGTTCCAGACATGTTT | 5’GTGACTGCCCAAGATGAAGACCAA | 600 | 5’TCCATCACTGATGATGACCTGGAGGCCATC |
| IL-1β | 5’ATGATGGCTTATTACAGTGGCAAT | 5’TTCACCATGCAATTTGTGTCTTCC | 777 | 5’ATGGAGCAACAAGTGGTGTTCTCCATG |
| IL-2 | Stratagene | Stratagene | 457 | 5’CCTCTGGAGGAAGTGCTAA |
| IL-3 | Stratagene | Stratagene | 449 | 5’CCATATCAAGGACGGTGACT |
| IL-4 | 5’CTGCTTCCCCCTCTGTTCTT | 5’CTGTGAAGGAAGCCAACCAG | 378 | 5’ACAGACATCTTTGCTGCCTC |
| IL-5 | Stratagene | Stratagene | 295 | 5’TTCAGGGAATAGGCACACTG |
| IL-6 | 5’AGTTGCCTTCTCCCTGG | 5’TGAGGGCTCTTCGGCAAAT | 621 | 5’CCAGCCTGCTGACGAAGCTGCAGGCACAG |
| IL-7 | 5’TTTTATTCCGTGCTGCTCGC | 5’GGTCAAAACGGATTAGGGCA | 430 | 5’TGGAATAAAATTTTGATGGGCACTAAAGAACACTGA |
| IL-8 | 5’ATGACTTCCAAGCTGGCCGTGGCTCTCTTG | 5’CTTACCTCACAGTGATGTTGTGAGGACATG | 886 | 5’GAGAAGTTTTTGAAGAGGGCTGAGAATTCA |
| IL-9 | 5’ATGCTTCTGGCCATGGTCCTTACCTCTGCCCTG | 5’GGGATGAGAGGCAAGATATGAAGATGAAATATT | 476 | 5’CAGTTGTCTCTGTTTGGGCA |
| IL-10 | 5’ATGCCCCAAGCTGAGAACCAAGACCCA | 5’TGGGATAGCTGACCCAGCCCCTT | 352 | 5’ACAATGAAGATACGAAACTGAGACATCAGG |
| IL-12 p40 | 5’GGATGCCCCTGGAGAAATGG | 5’AGGTGGAGGTCAGCTGGGAG | 655 | 5’TGCTGGTGGCTGACGACAAT |
| IL-12 p35 | Stratagene | Stratagene | 870 | 5’GCCCTGTGCCTTAGTAGTAT |
| IL-13 | 5’GCTCTTGCTTGCCTTGGTGG | 5’AAGCAACTGTTTCGCCACGG | 359 | 5’GCCATCTACAGGACCCAGAG |
| IL-15 | 5’CTCCCTAAAACAGAAGCCAAC | 5’GCAAAGAATGTGAGGAACTGG | 292 | 5’TGAAGTGCTTTCTCTTGGAG |
| IL-18 | 5’TTCGGGAAGAGGAAAGGAAC | 5’AAGGATACAAAAAGTGACAT | 480 | 5’GACTGATTCTGACTGTAGAG |
| TNFα | 5’GTTCCTCAGCCTCTTCTCCT | 5’ATCTATCTGGGAGGGGTCTT | 507 | 5’ACCCCGAGTGACAAGCCTGTAGCCCATGTT |
| TGFβ | 5’AAGCAGAGTACACACAGCATATATATGTTC | 5’ATTTGGAGCCTGGACACGCAGTACAGCAAG | 645 | 5’CTTCTCATGGACACCCCGCTGGAGAGGGCC |
| M-CSF | 5’GCAGGAGTATCACCGAGGAG | 5’CTCCCTCTTGCCTGGTGAG | 633 | 5’AGATAACACCCCCAATGCCA |
| GM-CSF | 5’AGCCCCAGCACGCAGCCCTGG | 5’CCCTTTGACTGCTGGGAGCCAGTCCAGGAG | 363 | 5’CGGGGCAGCCTCACCAAGCTCAAGGGCCC |
| IFNγ | 5’CTGTTACTGCCAGGACCCATATGTAAAAG | 5’CAAGGTCGAAGAGCATCCCAGTAATGGTTG | 448 | 5’GCGGATAATGGAACTCTTTTCTTAGGCAT |
| E-cadherin | 5’CCGATTCAAAGTGGGCACAG | 5’GCCATCGCTTACACCATCCT | 696 | 5’CCCCCTGTTGGTGTCTTTAT |
| Factor XIIIa | 5’TGTCAGAAACTTCCAGGACC | 5’CTGGACTGGAAGCGTTGACA | 954 | 5’GCAGATTGACTTCAGTCGT |
Southern blot analysis
All PCR products obtained were hybridized with a specific internal digoxygenin (DIG)-labeled probe (Boehringer Mannheim Corp., Mannheim, Germany) (Table I). Revelation of DIG-labeled cDNA on nylon membranes was performed as decribed by the manufacturer (Boehringer Mannheim Corp.).
Intracellular IL-10 production studies by flow cytometry
Intracellular IL-10 was detected by flow cytometry using the method of Andersson et al. (34) with modifications (35). DC cultured for 10 days were stimulated by irradiated CD40L L cells for 24 h (1 CD40L L cell for 5 DC), and submitted to Brefeldin A (10 μg/ml) for 5 h. Then cells were washed in PBS, fixed 15 min at room temperature in Fixation Medium A (Fix and Perm cells permeabilization kit; Caltag Laboratories, Burlingame, CA), washed twice with PBS, and incubated with FITC-labeled CD1a (Ortho Diagnostic Systems) and PE-labeled anti-IL-10 mAb (PharMingen, San Diego, CA) in the presence of permeabilization Medium B (Caltag Laboratories) for 15 min at 4°C. To compete IL-10 production, PE-labeled anti-IL-10 mAb was incubated previously with an excess of exogenous hIL-10 (100 μg/ml) (Schering-Plough Research Institute) for 30 min. Then cells were washed twice in PBS, resuspended in 1% formaldehyde (Sigma Chemical Co.), and analyzed using a FACScan flow cytometer (Becton Dickinson).
Cytokine assays
DC cultured for 12 days were stimulated by PMA-ionomycin for 6 h either by irradiated CD40L or control L cells for 24 h (1 CD40L L cell for 5 DC). Supernatants from 1.106 DC were assayed for cytokine production. The production of IL-10 was measured in culture supernatants by IL-10-specific ELISA (sensitivity, 100 pg/ml) using two rat mAbs kindly provided by Dr. J. S. Abrams (DNAX, Palo Alto, CA) (36). IL-8 production was measured by IL-8-specific immunoenzymetric assay (Medgenix Diagnostics S.A., Fleurus, Belgium) (sensitivity, 0.7 pg/ml). IL-13 production was measured by IL-13-specific ELISA (BioSource International, Camarillo, CA) (sensitivity, <12 pg/ml).
Results
RT-PCR demonstrates purification to homogeneity of two distinct subpopulations of DC generated in vitro from cord blood CD34+ progenitor cells
Human cord blood CD34+ hemopoietic progenitors cultured in the presence of GM-CSF and TNF-α differentiate into DC along two distinct pathways (24). Mutually exclusive expression of CD1a or CD14 at day 6 of culture allowed the isolation, after two rounds of FACS sorting, of pure populations of CD1a+ and CD14+ DC precursor cells. These DC precursor cells were then recultured in the presence of GM-CSF and TNF-α for 6 additional days. cDNA generated from the different samples were normalized according to the results of semiquantitative PCR amplification of β-actin (Fig. 1). At day 12, differentiation of both precursor subsets into mature DC with typical morphology and phenotype (CD1a+, CD80+, CD86+, high HLA class II) was confirmed by PCR amplification of CD1a, CD83, and CD86 mRNA. Only the CD1a+ subset differentiates into Langerhans cells characterized by the presence of Birbeck granules and the expression of E-cadherin. Semiquantitative analysis of E-cadherin by PCR showed a strong signal after 28 cycles of amplification in CD1a+-purified DC, while a weak band was detected only after 35 and 40 cycles in CD14+-purified DC (Fig. 1). The CD14+ subset has been characterized previously by the distinctive expression of intracytoplasmic CD68 and coagulation factor XIIIa, which are present in dermal DC, but not in epidermal Langerhans cells. Both factor XIIIa and CD68 mRNA expression were found to be restricted to purified CD14+ DC: a strong signal was observed after 35 cycles of PCR-derived amplification using CD14+-derived DC cDNA as template. In contrast, no message could be detected in purified CD1a+-derived DC even after hybridizing a 40-cycle PCR product with factor XIIIa-specific (Fig. 1) or CD68-specific (data not shown) probes. Semiquantitative RT-PCR analysis therefore confirmed the differential expression of specific markers by the two subsets of DC and established the lack of cross-contaminating cells.
E-cadherin mRNA characterizes CD1a+-derived DC, while factor XIIIa mRNA is expressed exclusively by CD14+-derived DC. Semiquantitative RT-PCR analysis of E-cadherin, factor XIIIa, and β-actin mRNA was performed on unsorted DC, and on CD1a+- or CD14+-derived DC. Specificity of E-cadherin and factor XIIIa mRNA was confirmed using an internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
E-cadherin mRNA characterizes CD1a+-derived DC, while factor XIIIa mRNA is expressed exclusively by CD14+-derived DC. Semiquantitative RT-PCR analysis of E-cadherin, factor XIIIa, and β-actin mRNA was performed on unsorted DC, and on CD1a+- or CD14+-derived DC. Specificity of E-cadherin and factor XIIIa mRNA was confirmed using an internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
Both CD1a+- and CD14+-derived DC constitutively express IL-7 mRNA and a large array of cytokine mRNAs
The presence of cytokine transcripts in the two purified DC subsets was compared by semiquantitative RT-PCR, using both resting cells and cells activated by either PMA-ionomycin (6 h) or CD40L-transfected L cells (24 h). For each cytokine, a positive control was amplified from cDNA of PMA-ionomycin-activated PBMC, except for IL-15, which was amplified from elutriated monocytes. Before studying the DC subsets, we analyzed the pattern of cytokines in the bulk DC population. None of the samples contained mRNA for IL-2, IL-3, IL-4, IL-5, IL-9, and IFN-γ, neither constitutively nor after activation. The absence of contaminating T cells was established further by the lack of PCR amplification of CD3 transcripts in any DC sample (data not shown). The numerous cytokine mRNAs detected in unsorted DC are listed in Table II.
Cytokine transcripts expressed by unsorted DC and purified CD1a+ and CD14+-derived DCa
| . | Unsorted DC . | CD1a+ . | CD14+ . |
|---|---|---|---|
| IL-1α | + | Low | + |
| IL-1β | + | Low | + |
| IL-6 | + | + | + |
| IL-7 | + | Low | + |
| IL-8 | + | + | + |
| IL-10 | + | − | + |
| IL-12 p40 | + | + | + |
| IL-12 p35 | + | + | + |
| IL-13b | + | + | + |
| IL-15 | + | + | + |
| IL-18 | + | + | + |
| TNFα | + | + | + |
| TGFβ | + | + | + |
| M-CSF | + | + | + |
| GM-CSFb | + | + | + |
| . | Unsorted DC . | CD1a+ . | CD14+ . |
|---|---|---|---|
| IL-1α | + | Low | + |
| IL-1β | + | Low | + |
| IL-6 | + | + | + |
| IL-7 | + | Low | + |
| IL-8 | + | + | + |
| IL-10 | + | − | + |
| IL-12 p40 | + | + | + |
| IL-12 p35 | + | + | + |
| IL-13b | + | + | + |
| IL-15 | + | + | + |
| IL-18 | + | + | + |
| TNFα | + | + | + |
| TGFβ | + | + | + |
| M-CSF | + | + | + |
| GM-CSFb | + | + | + |
The presence of a specific PCR product is represented by a positive signal (+), whereas no detection of PCR product after Southern blot hybridization is represented by a negative signal (−). “Low” means that the specific PCR product was faint even after Southern blot hybridization. Cells were recovered at day 12 and IL-2, IL-3, IL-4, IL-5, IL-9 and IFNγ were not detected on total cells. Results are representative of at least four independent cell preparations analyzed without further activation.
PMA-ionomycin activation.
Cytokine expression was next analyzed in the two highly purified DC subpopulations. Among a large panel of cytokines tested, mRNAs for IL-1α, IL-1β, IL-6, IL-15, TNF-α, TGF-β, and macrophage CSF were amplified from unstimulated DC from both subsets (Table II). However, IL-1α and IL-1β signals were consistently lower in CD1a+DC than in CD14+-derived DC. GM-CSF mRNA was expressed in both DC subsets, but only after PMA-ionomycin activation.
IL-7 mRNA was detected in both DC subsets, albeit at a different level: a specific PCR product was detected after 40 and 28 cycles of amplification in CD1a+ and CD14+-derived DC, respectively (Fig. 2). Specificity of IL-7 amplification was confirmed by sequencing the PCR product (data not shown). However, secretion of the protein could not be observed using an IL-7-specific ELISA, a possible consequence of IL-7 release below detection levels.
Both DC subpopulations express IL-7 mRNA. Semiquantitative RT-PCR analysis of IL-7 mRNA was performed on unsorted DC and on CD1a+- or CD14+-derived DC. Specificity of IL-7 mRNA was confirmed using a specific internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
Both DC subpopulations express IL-7 mRNA. Semiquantitative RT-PCR analysis of IL-7 mRNA was performed on unsorted DC and on CD1a+- or CD14+-derived DC. Specificity of IL-7 mRNA was confirmed using a specific internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
Both DC subpopulations express IL-12 p35, p40, and IL-18 mRNA and secrete IL-12
DC and Langerhans cells were shown previously to secrete IL-12, a heterodimeric cytokine composed of two covalently linked (p40 and p35) chains (11, 14, 37), and this secretion is enhanced by CD40 ligation (12, 13). Consistent with these reports, IL-12 p40 and IL-12 p35 mRNA were constitutively present in both DC subsets (Fig. 3,A), and their expression was up-regulated after either CD40L or PMA-ionomycin activation. Moreover, a p70 IL-12-specific ELISA detected the protein both in CD1a+ and CD14+ subset supernatants, but only after CD40L activation (CD1a+, 0.1 ± 0.08 ng/ml, mean of n ≥ 10, range 0.03–0.29; CD14+, 0.11 ± 0.06 ng/ml, mean of n ≥ 10, range 0.03–0.19). Interestingly, both DC subpopulations also expressed mRNA for IL-18 (or IFN-γ-inducing factor), a recently described cytokine produced by monocyte and macrophage cell lines (38), and which shares some biologic activities with IL-12 (Fig. 3 B).
Both DC subpopulations express IL-12 p35, p40, and IL-18 mRNA. A, IL-12 p35 and p40 mRNA expression by unsorted DC, and by CD1a+- or CD14+-derived DC was analyzed using semiquantitative RT-PCR on unactivated cells, PMA-ionomycin, or CD40L-activated cells. Specificity of IL-12 p40 mRNA was confirmed using a specific internal DIG-labeled probe. B, IL-18 mRNA expression by unsorted DC, and by CD1a+- or CD14+-derived DC was analyzed using semiquantitative RT-PCR on unactivated cells, PMA-ionomycin, or CD40L-activated cells. Specificity of IL-18 mRNA was confirmed using a specific internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
Both DC subpopulations express IL-12 p35, p40, and IL-18 mRNA. A, IL-12 p35 and p40 mRNA expression by unsorted DC, and by CD1a+- or CD14+-derived DC was analyzed using semiquantitative RT-PCR on unactivated cells, PMA-ionomycin, or CD40L-activated cells. Specificity of IL-12 p40 mRNA was confirmed using a specific internal DIG-labeled probe. B, IL-18 mRNA expression by unsorted DC, and by CD1a+- or CD14+-derived DC was analyzed using semiquantitative RT-PCR on unactivated cells, PMA-ionomycin, or CD40L-activated cells. Specificity of IL-18 mRNA was confirmed using a specific internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
Activated CD14+-derived DC, but not CD1a+-derived DC, secrete IL-10
IL-10 is a cytokine produced by numerous cell types, including T cells, activated B cells, monocytes/macrophages, basophils/mast cells, and keratinocytes, but to date, no production of IL-10 protein by DC has been reported (39). Using semiquantitative RT-PCR analysis, total DC were found to transcribe IL-10 mRNA (Table II). Remarkably, a specific IL-10 PCR product was detected in unactivated CD14+-derived DC after 40 cycles, but not in unactivated CD1a+-derived DC, even after Southern blot hybridization (Fig. 4). The exclusive expression of IL-10 mRNA by CD14+-derived DC was confirmed after activation with either CD40L or PMA-ionomycin, which both up-regulated IL-10 mRNA expression in CD14+-derived DC, whereas IL-10 transcripts remained undetectable in CD1a+-derived DC. IL-10 protein production was next measured by ELISA. As shown in Figure 5 A, low levels of IL-10 were produced constitutively by unsorted DC (0.16 ± 0.3 ng/ml, mean of n ≥ 10, range <0.05–0.7). Up to 0.367 ng/ml of IL-10 was produced spontaneously by CD14+-purified DC, while no IL-10 was detected in the supernatant of purified CD1a+-derived DC (n ≥ 10). IL-10 production was also analyzed after PMA-ionomycin or CD40L activation, which induced a strong secretion of IL-8 in the supernatants (24 ± 8.2 ng/ml, mean of n ≥ 10, range 13.1–43.4, and 11.6 ± 7.6 ng/ml, mean of n ≥ 10, range 4.4–25.3, respectively). In line with RNA data, spontaneous secretion of IL-10 by unpurified DC was enhanced significantly following CD40L activation (0.6 ± 0.5 ng/ml, mean of n ≥ 10, range 0.2–1.7) and was restricted strictly to the CD14+-derived cells that secreted up to 2.14 ng/ml (0.8 ± 0.6 ng/ml, mean of n ≥ 10, range 0.2–2). However, PMA-ionomycin activation did not up-regulate, but rather decreased the production of IL-10.
CD14+-derived DC, but not CD1a+-derived DC, produce IL-10 mRNA. IL-10 mRNA expression of unsorted DC, and of CD1a+- or CD14+-derived DC was analyzed using semiquantitative RT-PCR on unactivated cells, PMA-ionomycin, or CD40L-activated cells. Specificity of IL-10 mRNA was confirmed using a specific internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
CD14+-derived DC, but not CD1a+-derived DC, produce IL-10 mRNA. IL-10 mRNA expression of unsorted DC, and of CD1a+- or CD14+-derived DC was analyzed using semiquantitative RT-PCR on unactivated cells, PMA-ionomycin, or CD40L-activated cells. Specificity of IL-10 mRNA was confirmed using a specific internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
Only CD14+-derived DC produce IL-10. A, The production of IL-10 (▪) by unsorted DC, and by CD1a+- or CD14+-derived DC was measured at day 12 of the culture using a specific ELISA, with supernatants from unactivated cells, PMA-ionomycin, or CD40L-activated cells. To control the efficiency of activation, comparatively to IL-10 production, IL-8 secretion (▨) was measured by a specific ELISA in the samples tested. Results, expressed in ng/ml, represent one experiment, representative of 10. In the experiment shown, unactivated or CD40L-activated DC produced an average 0.278 and 0.475 ng/ml of IL-10, respectively, whereas unactivated or CD40L-activated CD14+-derived cells produced 0.223 and 1.57 ng/ml, respectively. B, The production of IL-10 by unsorted DC was analyzed by intracellular flow cytometry at day 10 of the culture after 24-h stimulation by irradiated CD40L L cells. CD1a expression was used as a DC marker recognizing both CD1a+- and CD14+-derived cells. To compete IL-10 production, PE-labeled anti-IL-10 mAb was incubated previously with an excess of exogenous hIL-10 (100 μg/ml).
Only CD14+-derived DC produce IL-10. A, The production of IL-10 (▪) by unsorted DC, and by CD1a+- or CD14+-derived DC was measured at day 12 of the culture using a specific ELISA, with supernatants from unactivated cells, PMA-ionomycin, or CD40L-activated cells. To control the efficiency of activation, comparatively to IL-10 production, IL-8 secretion (▨) was measured by a specific ELISA in the samples tested. Results, expressed in ng/ml, represent one experiment, representative of 10. In the experiment shown, unactivated or CD40L-activated DC produced an average 0.278 and 0.475 ng/ml of IL-10, respectively, whereas unactivated or CD40L-activated CD14+-derived cells produced 0.223 and 1.57 ng/ml, respectively. B, The production of IL-10 by unsorted DC was analyzed by intracellular flow cytometry at day 10 of the culture after 24-h stimulation by irradiated CD40L L cells. CD1a expression was used as a DC marker recognizing both CD1a+- and CD14+-derived cells. To compete IL-10 production, PE-labeled anti-IL-10 mAb was incubated previously with an excess of exogenous hIL-10 (100 μg/ml).
Furthermore, we analyzed IL-10 production on day 10 cultured DC at single cell level by measuring intracellular production of IL-10 by flow cytometry. As shown in Figure 5 B, CD40-activated CD1a+ DC produced IL-10, and an excess of exogenous hIL-10 completely prevented IL-10 detection. At that stage of differentiation, CD1a stained both CD1a+- and CD14+-derived DC. Day 10 DC were used to optimize intracytoplasmic IL-10 detection, as IL-10 is best visualized at immature stage.
Taken together, these results demonstrate that CD14+-derived DC (expressing CD1a) produce IL-10. This restriction of IL-10 secretion to the CD14+-derived DC subpopulation is likely to result in functional differences between the two subsets.
In contrast to IL-10 and IL-12, PMA-ionomycin, but not CD40 activation, induces IL-13 production
Unlike many other cytokines, human IL-13 and IL-4 are produced by relatively few cell types, including activated T cells, mast cells, and basophils (40, 41, 42, 43, 44, 45). Surprisingly, high amounts of IL-13 transcripts were found in both DC subsets following PMA-ionomycin activation (Table II and Fig. 6), but not after CD40L activation. In contrast, no IL-4 transcript could be detected even after activation. The absence of CD3, IL-3, IL-4, and IL-5 mRNA in the highly purified CD1a+ and CD14+ DC subsets (data not shown) argues against contaminating T cells or basophils being the source of the IL-13 signal. No IL-13 secretion was detected by ELISA in supernatants of either resting DC subsets (Fig. 7). Consistent with PCR results, activation of total DC by PMA-ionomycin resulted in detectable secretion of IL-13 (0.12 ± 0.09 ng/ml, mean of n ≥ 10, range 0.03–0.36). Both CD1a+- and CD14+-derived cells produced significant amounts of IL-13 (0.46 ± 0.54 ng/ml, mean of n ≥ 10, range 0.04–1.38, and 0.8 ± 0.32 ng/ml, mean of n ≥ 10, range 0.08–1.25, respectively), in response to PMA-ionomycin, but failed to respond to CD40L activation, contrasting with IL-10 and IL-12 productions. Taken together, these data suggest that an activation signal distinct from CD40/CD40L triggers both DC subsets to secrete IL-13, but not IL-4.
Both DC subpopulations express IL-13 mRNA following PMA-ionomycin activation. IL-13 mRNA expression by unsorted DC, and by CD1a+- or CD14+-derived DC was analyzed using semiquantitative RT-PCR on unactivated cells, PMA-ionomycin, or CD40L-activated cells. Specificity of IL-13 mRNA was confirmed using a specific internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
Both DC subpopulations express IL-13 mRNA following PMA-ionomycin activation. IL-13 mRNA expression by unsorted DC, and by CD1a+- or CD14+-derived DC was analyzed using semiquantitative RT-PCR on unactivated cells, PMA-ionomycin, or CD40L-activated cells. Specificity of IL-13 mRNA was confirmed using a specific internal DIG-labeled probe. Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
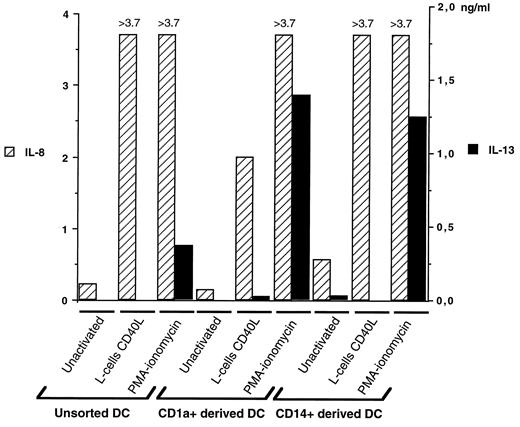
PMA-ionomycin-activated DC subpopulations secrete IL-13. The production of IL-13 (▪) by unsorted DC, and by CD1a+- or CD14+-derived cells was measured at day 12 of the culture using a specific ELISA, with supernatants from unactivated cells, PMA-ionomycin, or CD40L-activated cells. To control the efficiency of the activation, comparatively to IL-13 production, IL-8 secretion (▨) was measured by a specific ELISA in the samples tested. Results, expressed in ng/ml, represent one experiment, representative of 10. In the experiment shown, PMA-ionomycin-activated DC cells produced an average 0.361 ng/ml, whereas PMA-ionomycin-activated CD1a+- or CD14+-derived cells produced 1.384 and >1.250 ng/ml IL-13, respectively.
PMA-ionomycin-activated DC subpopulations secrete IL-13. The production of IL-13 (▪) by unsorted DC, and by CD1a+- or CD14+-derived cells was measured at day 12 of the culture using a specific ELISA, with supernatants from unactivated cells, PMA-ionomycin, or CD40L-activated cells. To control the efficiency of the activation, comparatively to IL-13 production, IL-8 secretion (▨) was measured by a specific ELISA in the samples tested. Results, expressed in ng/ml, represent one experiment, representative of 10. In the experiment shown, PMA-ionomycin-activated DC cells produced an average 0.361 ng/ml, whereas PMA-ionomycin-activated CD1a+- or CD14+-derived cells produced 1.384 and >1.250 ng/ml IL-13, respectively.
Ex vivo isolated GCDC express IL-7, IL-10, and IL-13
The physiologic relevance of IL-7, IL-10, and IL-13 expression by DC was evaluated on DC isolated ex vivo. CD4+CD11c+CD3− DC (GCDC) that strongly express class II Ags, but are CD1a− and do not contain Birbeck granules (31), were purified from tonsillar germinal centers. The absence of T and B cell contaminations in purified GCDC was established by the absence of CD3 and CD19 PCR products (Fig. 8, lanes 3, 4, 7, and 8). Similar levels of IL-7 transcription were detected in GCDC with or without PMA-ionomycin activation (lanes 11 and 12). IL-10 mRNA, which was detected in unactivated GCDC (lane 15), was up-regulated following PMA-ionomycin (lane 16) or CD40L activation (data not shown). Similar to in vitro generated DC, GCDC expressed IL-13 mRNA only following PMA-ionomycin activation (lane 20), and not after CD40L triggering (data not shown). Therefore, production of IL-7, IL-10, and IL-13, which is not limited to DC generated in vitro, may have physiologic consequences on in vivo immune response. In addition, similar cytokine production patterns raise the possibility that GCDC are related to the CD14+-derived DC generated in vitro.
Expression of IL-7, IL-10, and IL-13 mRNA by germinal center DC isolated ex vivo. CD3, CD19, IL-7, IL-10, and IL-13 mRNA expression in unactivated and PMA-ionomycin-activated GCDC was analyzed by RT-PCR. Specificity of each mRNA amplification was confirmed using a specific internal DIG-labeled probe (data not shown). Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
Expression of IL-7, IL-10, and IL-13 mRNA by germinal center DC isolated ex vivo. CD3, CD19, IL-7, IL-10, and IL-13 mRNA expression in unactivated and PMA-ionomycin-activated GCDC was analyzed by RT-PCR. Specificity of each mRNA amplification was confirmed using a specific internal DIG-labeled probe (data not shown). Negative control (−) was performed by omitting cDNA. PMA-ionomycin-stimulated normal human PBMC mRNA was used as a positive control template (+). Results are representative of at least four independent cell preparations analyzed.
Discussion
We have studied herein the pattern of cytokines expressed by various DC populations either generated in vitro or isolated from tonsils. The analysis was concentrated initially on CD1a+- and CD14+-derived DC, two distinct DC subpopulations generated in vitro by culturing CD34+ progenitors with GM-CSF and TNF-α. The results demonstrate that 1) both populations of DC produce a large array of cytokines known to contribute to T cell priming (IL-1α, IL-6, IL-15, TNF-α) or to T cell maturation (IL-12, IL-18, IL-7); 2) different signals lead to a different regulation of the production of immunologically relevant cytokines (IL-12, IL-13); 3) only CD14+-derived DC produce IL-10, a key immunomodulator; 4) finally, DC isolated ex vivo (GCDC) express IL-7, IL-10, and IL-13 and thus may represent derivatives of the CD14+ interstitial lineage.
Among the various cytokines tested, both subsets express transcripts for IL-1α, IL-1β, IL-6, IL-15, TNF-α, and TGF-β, cytokines that potentially regulate naive T cell activation. Both CD1a+- and CD14+-derived cells expressed IL-1α and IL-1β transcripts, although transcription levels were lower in the CD1a+-derived DC. This is in agreement with the reported expression of IL-1α and IL-1β mRNA within Langerhans cells and peripheral blood DC (17, 46, 47, 48). The secretion of IL-1β by DC, and in particular by Langerhans cells, seems to be essential for induction of primary immune responses in skin (10). IL-15, a recently identified cytokine with IL-2-like properties, was shown to increase Ag-specific T cell activity (49). The expression of IL-15 mRNA in both DC subsets is in line with description of this cytokine in Langerhans cells and in human blood-derived DC (50). Furthermore, human blood DC were reported to produce functional IL-15 protein with chemotactic activity for T cells (19). Both DC subsets were found to express IL-6, TNF-α, TGF-β, macrophage CSF, and GM-CSF (only following activation) transcripts that may regulate the capacity of DC to initiate an immune response.
IL-12 is a heterodimeric molecule produced by APCs that appears to be central in promoting Th1 differentiation through induction of IFN-γ production (51). It is not yet clear whether IL-12 p35 and p40 chains are expressed constitutively in APCs, but both chains need to be assembled to form biologically active IL-12 protein (37). In our hands, the two constitutively expressed chains were up-regulated following CD40L activation, and IL-12 protein was detectable in both DC subsets, although only after CD40L stimulation. Our results are in agreement with a previous study reporting constitutive IL-12 p40 and p35 mRNA expression in DC, and secretion of an active IL-12 p70, up-regulated by Staphylococcus aureus Cowan I stimulation (15) or CD40 triggering (12, 13). Furthermore, both DC subpopulations were found to express IL-18 (or IGIF) transcript. IL-18 is a recently identified cytokine that synergizes with IL-12 to induce the production of IFN-γ by Th cells (38, 52) and to block IgE production from B cells (53). It is therefore not unlikely that the large amounts of IFN-γ secreted by T cells activated by DC result from the cooperation between DC-derived IL-12 and IL-18.
In contrast to the results of a previous study (17) using mature blood DC, we report in this work constitutive expression of IL-7 mRNA in both DC subsets analyzed. IL-7 was shown originally as a bone marrow stromal cell-derived cytokine supporting growth of B cell (54, 55) and T cell precursors (56). It also has been detected in human follicular DC (57), but was never observed in cells of hemopoietic origin. The finding that DC express IL-7 is of interest in regard to the recently identified role of IL-7 in naive CD4+ T cell activation, early IL-4 secretion, and commitment toward Th2 development (58). Furthermore, in mice, IL-7 appears to regulate the functional development of an MHC class I-like restricted NK.1.1 T cell subset (59) that is involved in Th2 commitment through production of high levels of IL-4 (60, 61, 62).
Of interest, both DC subpopulations presently analyzed secreted IL-13, but not IL-4, upon PMA-ionomycin stimulation. Both cytokines are known to be secreted mostly by the same cell types, including activated T cells (40, 41), mast cells, and basophils (42, 43, 44, 45). However, differences in cellular sources have been reported inasmuch as IL-13 is produced by CD4+ and CD8+ T cell clones belonging to Th0, Th1, and Th2 subsets (de Waal Malefyt, (63)), while IL-4 is secreted mostly by Th0, Th2-like, and NK1.1+CD4+ T cells (60, 64). Moreover, contrary to eosinophils producing IL-4, but not IL-13, malignant and EBV-transformed B cells only produce IL-13 (63, 65, 66). DC-derived IL-13 might participate in several aspects of DC functions: 1) as it can substitute for IL-4, IL-13 might have an autocrine function during differentiation of CD14+ precursors into DC; 2) by strongly inhibiting the production of proinflammatory cytokines, such as IL-1α, IL-1β, IL-6, IL-8, IL-10, GM-CSF, TNF-α (67), and IL-12, IL-13 could indirectly favor the commitment of naive T cells toward the Th2 pathway; 3) DC-derived IL-13 is likely to contribute to the regulation of B cell proliferation and differentiation by DC (68, 69, 70).
Of importance, only PMA-ionomycin activation up-regulates IL-13, GM-CSF secretion, and lymphotactin mRNA expression (data not shown), while only CD40 triggering induces IL-10 and IL-12 production. This differential response suggests the existence of DC activation signal(s) distinct from CD40/CD40L, the surface triggers of which remain to be identified.
Of particular interest, we found that only CD14+-derived DC cells can produce IL-10, either constitutively at a low level, or in larger amounts after CD40L activation. In this context, monocyte-derived DC (data not shown) as well as blood DC (17) express IL-10, while Langerhans cells fail to express IL-10 mRNA (71). The unique capacity of CD14+-derived cells to produce IL-10 strongly supports the concept that these two populations represent two independent pathways of DC development (24, 28), and suggests a particular role of this DC subset in the priming of naive T cells. Thus, IL-10 might be involved in controlling the levels of T cell activation induced by DC (72). Furthermore, IL-10 has been shown recently to directly act on T cells to induce a state of anergy (73). Thus, CD14+-derived DC might have a specific role in the induction of T cell tolerance through the production of IL-10. Moreover, by analogy to IL-10 effects on monocytes (74), endogenous IL-10 may down-regulate the production of IL-1α, IL-1β, IL-6, IL-8, TNF-α, and GM-CSF by DC.
Another important aspect of this study is the correlation between cytokine expression of DC generated in vitro and DC isolated ex vivo. Thus, IL-7, IL-10, and IL-13 mRNA expression was observed on a recently identified ex vivo purified CD4+CD11c+CD3− DC population (GCDC) (31). GCDC are in close contact with T cells and B cells in germinal centers, suggesting an important role of these cytokines during B cell responses in vivo. Like CD14+-derived DC generated in vitro, GCDC express IL-10 and factor XIIIa mRNA (data not shown), but lack Birbeck granules, CD1a, CD40, CD80, CD83, and CD86 molecules. In this context, CD14+-derived DC may be related to GCDC, and thus represent an interesting model to study in vitro DC functions.
In conclusion, human DC secrete a large array of soluble factors, including several cytokines and growth factors that are immunologically relevant. IL-7, IL-10, and IL-13 were found unexpectedly to be expressed by DC, and the role of these cytokines during DC-T and DC-B cell interactions remains to be established. Taken together, our findings indicate that, depending on the subset of cells considered and on the condition of activation, human DC can produce different sets of cytokines. It is therefore likely that the outcome of a primary immune response will be affected not only by the subset of DC involved, but also by the activation signal engaged during the initiation phase of the response.
Acknowledgements
We are grateful to H. Yssel, D. Blanchard, and S. Saeland for critically reading the manuscript; D. Blanchard for helpful discussions; E. Garcia and I. Durand for FACS sorting; S. Bonnet-Arnaud for editorial assistance; doctors and colleagues from hospitals in Lyon who provide us with umbilical cord blood samples; and Dr. J. Chiller for support and for discussions.
Footnotes
Preliminary results were presented at Fourth International Symposium on Dendritic Cells in Fundamental and Clinical Immunology, held in Venice (Italy) in October 1996.
Abbreviations used in this paper: DC, dendritic cell; CD40L, CD40 ligand; DIG, digoxygenin; GCDC, germinal center dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; h, human; PE, phycoerythrin; RT-PCR, reverse-transcriptase-polymerase chain reaction; SCF, stem cell factor.