Abstract
CC chemokine receptor 5 (CCR5) functions physiologically as a receptor for the leukocyte chemoattractants macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, and RANTES, and functions pathologically as a key cell entry coreceptor for HIV-1. The factors that regulate CCR5 expression may be useful therapeutic targets for HIV-1 infection. To identify nuclear regulatory factors, we have located and functionally characterized the CCR5 gene promoter. The gene consists of two exons separated by a 1.9-kb intron. Exon 1 contains 43 bp of the 5′-untranslated region; exon 2 contains 11 bp of the 5′-untranslated region and the complete open reading frame. Primer extension analysis identified two adjacent transcriptional start points (tsp) that map to the first 2 bp found in the longest known CCR5 cDNA sequence. A TATA box is present 31 bp upstream from the first tsp. CCR5 mRNA was detected constitutively in both primary human myeloid and lymphoid cells by Northern blot hybridization. Consistent with this, transcription of a chloramphenicol acetyltransferase reporter gene was constitutively activated in both transiently transfected myeloid and lymphoid cell lines by the 80-bp gene fragment located immediately upstream of the tsp. Deletion analysis located a strong silencer element between nucleotides −244 and −80, and a strong enhancer element between −486 and −244. These results suggest that the gene region between −486 and −1 may regulate the expression of CCR5 in monocyte/macrophages and T lymphocytes.
Chemokines constitute a structurally related family of secreted proteins, most of which chemoattract and activate specific subsets of leukocytes in vitro. Chemokines are classified into two major subfamilies depending on the position of the first two of four conserved cysteines, which are adjacent in the case of CC chemokines and separated by a single amino acid in the case of CXC chemokines (1). In vitro, CXC chemokines attract lymphocytes and neutrophils, whereas CC chemokines typically do not attract neutrophils, but instead attract monocytes, macrophages, eosinophils, basophils, dendritic cells, and lymphocytes with variable selectivity and potency. In vivo, chemokines appear to act as locally produced emergency signals that direct leukocytes to sites of infection and tissue injury, but they may also regulate basal leukocyte trafficking, hemopoiesis, angiogenesis, and other processes (2, 3, 4, 5).
Chemokines activate leukocytes by binding to selective, seven-transmembrane domain, G protein-coupled receptors present on the plasma membrane (6). To date, twelve functional human chemokine receptors have been identified: four are specific for CXC chemokines, and eight are specific for CC chemokines. Most of the receptors identified to date bind multiple chemokines; conversely, most chemokines tested to date bind to two or more receptor subtypes. In addition to their suspected beneficial role in host defense and tissue repair, several chemokine receptors (e.g., CCR2B, CCR3, CCR5, and CXCR4)4 are exploited pathologically by HIV-1, acting together with CD4 as cell entry coreceptors in vitro (7, 8, 9, 10, 11, 12, 13, 14). The HIV-1 strain specificity of the coreceptors is complex. CCR5 is used preferentially by most primary isolates and not by T cell line-adapted laboratory strains (7, 8, 9, 10, 11, 12, 13, 14, 15). CXCR4 is used preferentially by laboratory strains and by some primary isolates. CCR3 is used by both primary isolates and laboratory-adapted strains (11, 12, 16, 17). Only a few strains are able to use CCR2B (12). The importance of this for HIV-1 transmission in vivo has been clarified for CCR5 by the discovery of a benign, inherited, nonfunctional mutant CCR5 allele that in homozygous individuals is associated with a high level of resistance to natural HIV-1 infection (18, 19, 20, 21, 22). Consistent with this, the specific agonists for CCR5, macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, and RANTES, are able to suppress infection of CCR5-expressing cells by appropriate HIV-1 strains (8, 9, 10, 11, 12, 23). Also, HIV-1+ individuals heterozygous for the mutant CCR5 allele appear to have slightly delayed progression to AIDS compared with individuals homozygous for the wild-type allele (20, 21). This implies that measures designed to block CCR5 expression or function could be used to block HIV-1 transmission and/or to treat established HIV-1 infection. In this regard, detailed knowledge of the factors regulating CCR5 expression is an important goal.
CCR5 mRNA has been detected in PBMCs and adherent monocytes (8, 24). Using a specific mAb, CCR5 protein has been detected in microglial cells of the central nervous system and memory T cells (25, 26). CCR5 protein expression can be up-regulated by treatment of T cells with IL-2 (26). In contrast, CCR5 RNA and HIV-1 coreceptor function can be down-regulated in CD4+ T cells by CD3/CD28 costimulation (27). In the present report we have identified the structural organization and sequence of the CCR5 gene and have located a functional promoter with high resolution that may be responsible for constitutive expression of CCR5 in both myeloid and lymphoid tissues.
Materials and Methods
Cell culture
Derivation of human CD4+ and CD8+ tumor-infiltrating T lymphocytes (Til cells) has been previously described (28, 29). The cells were provided by J. Farber (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). The histiocytic lymphoma cell line U937, the acute T cell leukemia cell line Jurkat, and the human embryonic kidney (HEK) cell line 293 were obtained from the American Type Culture Collection (Rockville, MD). U937 and Jurkat cells were grown in RPMI 1640 (Biofluids, Rockville, MD) supplemented with 10% heat-inactivated FBS (HyClone (Logan, UT) or Life Technologies (Gaithersburg, MD)), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Quality Biologics, Gaithersburg, MD). CD4 and CD8 Til cells were grown in AIM-V medium with IL-2 (500 U/ml), glutamine, streptomycin (50 μg/ml), and gentamicin (10 μg/ml; Life Technologies) supplemented with 10% heat-inactivated FBS. HEK 293 cells were grown in DMEM (Biofluids) supplemented with 10% FBS, 4.5 g/l glucose, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cells were grown at 37°C in 5% CO2 in a humidified incubator.
Northern blot analysis of RNA
Total RNA was isolated from cultured cell lines and primary leukocytes using a kit (Qiagen (Chatsworth, CA) or Stratagene (La Jolla, CA)). PBMCs were purified from healthy human donors by Hypaque/Ficoll density gradient centrifugation, dextran sedimentation, and hypotonic lysis of residual erythrocytes. Lymphocytes and monocytes were separated by adherence of the mononuclear layer from the Hypaque/Ficoll gradient to tissue culture plastic in RPMI 1640 with 10% FBS at 37°C in 5% CO2 for 18 h. Lymphocytes were recovered in the nonadherent fraction of cells. Highly purified human monocytes were obtained by elutriation performed by the Department of Transfusion Medicine, National Institutes of Health. Isolated RNA (10 μg/lane) was electrophoresed in a 1% agarose gel containing 2% formaldehyde in 3-morpholino-propanesulfonic acid (MOPS) buffer (pH 7.0) consisting of 10 mM MOPS, 5 mM sodium acetate, and 1 mM EDTA. After migration, RNA was transferred overnight by capillary action onto Nytran membranes (Schleicher and Schuell, Keene, NH) and UV cross-linked using a Stratalinker (Stratagene). Blots were probed with the total open reading frame (ORF) of CCR5 labeled with [α-32P]dCTP using a random-primed DNA labeling kit (Boehringer Mannheim, Indianapolis, IN) and purified on size exclusion columns (Stratagene). The blots were prehybridized in a buffer containing 50% formamide, 6× SSPE, 0.5% SDS, and 50 μg/ml denatured salmon sperm DNA for 90 min at 37°C. The radiolabeled probe was added (1 × 106 cpm/ml), and the filters were hybridized overnight at 37°C. The filters were then washed with 1× SSPE and 0.1% SDS at 60°C for 30 min and autoradiographed with Kodak X-OMAT AR films (Eastman Kodak, Rochester, NY) between intensifying screens at −80°C.
RNA analysis by primer extension
Poly(A)+ RNA was purified from CD4 Til cell total RNA using the Poly(A) Quik Kit (Stratagene), and 1 μg was analyzed using a commercial primer extension kit according to the instructions of the manufacturer (Promega, Madison, WI). Briefly, an antisense CCR5-specific primer (10 pmol), corresponding to nucleotides 78 to 58 (5′-TGGACTTGACACTTGATAATC-3′) of the clone 134 cDNA encoding CCR5, reported by Raport et al. (30), was end-labeled with [γ-32P]ATP and annealed to the poly(A)+ RNA at 53°C for 20 min before reverse transcription using avian myeloblastosis virus reverse transcriptase. A control reaction was performed in parallel using control RNA provided in the kit. The reaction products were separated on a 6% acrylamide gel containing 8 M urea along with known DNA sequence for size determination. After electrophoresis, the gel was dried and visualized by autoradiography.
Genomic DNA analysis
CCR5 genomic clones were isolated by plaque hybridization from a commercially available human library in the vector λ FIX (Stratagene) using the 63-2 cDNA encoding a portion of the CCR5 ORF as a probe (31) labeled with [α-32P]dCTP by the random primed DNA labeling kit. Clones containing the 5′ end of the gene were identified by hybridization with two 5′-UTR sense primers corresponding to nucleotides 1 to 21 (5′-AGAAGAGCTGAGACATCCGTT-3′) and nucleotides 18 to 42 (5′-CGTTCCCCTACAAGAAACTCTCCC-3′) of the clone 134 CCR5 cDNA (30). The same probes were then used for mapping restriction sites and to identify appropriate restriction fragments for subcloning, sequencing, and functional analysis. DNA sequences were analyzed with software from the University of Wisconsin Genetics Computer Group on a Cray supercomputer maintained by the National Cancer Institute Advanced Scientific Computing Laboratory, Frederick Cancer Research and Development Center (Frederick, MD) (32).
Reporter gene constructs
The reporter gene used in these studies was bacterial chloramphenicol acetyltransferase (CAT) as found in the pCAT-basic expression vector (Promega). A 2.5-kb XbaI/EcoRI fragment containing the putative 5′ end of the CCR5 gene was subcloned into Bluescript KS II. Portions of this fragment were amplified by PCR using Pfu polymerase (Stratagene) and primers containing 21 specific nucleotides with additional 5′ nucleotides encoding either PstI or XbaI sites to facilitate subcloning upstream of CAT. The PCR conditions were denaturation at 94°C for 90 s, annealing at 59°C for 2 min, and extension at 72°C for 2 min (25 cycles). All constructs were confirmed by DNA sequencing on both strands. The pCAT-basic plasmid, which contains the CAT ORF without a promoter or an enhancer, was used as a negative control. pSV40 (pCAT-Promoter, Promega), which has the SV40 promoter cloned in the sense orientation upstream of CAT, was used as a positive control.
CAT assay
The human cell lines Jurkat and U937 were grown in suspension as described above to a density of 0.5 to 1.0 × 106 cells/ml, and adherent HEK 293 cells were grown to subconfluence. Cells were harvested and resuspended at a density of 30 × 106 cells/ml in their respective complete medium. Uncut plasmid DNA (20 μg; prepared with Qiagen Maxiprep kit) was used to electroporate 15 × 106 cells in 500 μl of complete medium with a 0.4-cm gap electroporation cuvette (Bio-Rad Laboratories, Hercules, CA) at 960 μF and 250 V using a Gene Pulser (Bio-Rad). The cells were then chilled on ice, added to 30 ml of complete medium, and incubated for 2 days at 37°C in 5% CO2 in a humidified incubator. In addition, cells were cotransfected with 10 μg of pCMV (CMV)-β-galactosidase plasmid (Clontech, Palo Alto, CA) as a control for electroporation efficiency. The level of β-galactosidase activity was determined spectrophotometrically using a β-galactosidase assay kit (Promega). In some experiments, plasmid DNA was transferred to target cells by lipofection. Specifically, 3 μg of test plasmid DNA plus 2 μg of pCMV-β-galactosidase plasmid DNA were mixed with 20 μl of Superfect (Qiagen), according to the instructions of the manufacturer, and then added to 5 million target cells in appropriate media. After 2 days of incubation, transfected cells were harvested by centrifugation at 1,800 rpm for 5 min, washed twice with PBS, and resuspended in 500 μl of a buffer containing 40 mM Tris (pH 7.4), 1 mM EDTA, and 150 mM NaCl. The cells were incubated for 5 min at room temperature, centrifuged at 14,000 rpm for 1 min, resuspended in 100 μl of 250 mM Tris, pH 7.8, and disrupted by freezing/thawing four times using dry ice and a 37°C water bath. Cell debris was removed by a 2-min centrifugation at 14,000 rpm. A portion of the supernatant containing 50 μg of protein was incubated overnight with 250 mM Tris (pH 7.5), 9 mM acetyl coenzyme A (Pharmacia Biotech, Piscataway, NJ), and 0.25 μCi of [14C]chloramphenicol (DuPont-New England Nuclear, Boston, MA) in a final volume of 150 μl at 37°C. The [14C]chloramphenicol and acetylated products were then extracted with 1 ml of ethyl acetate by vortexing for 30 s. The ethyl acetate layer was removed, lyophilized, resuspended in 30 μl of ethyl acetate, and applied to a TLC sheet (Baker-flex silica gel 1B, J. T. Baker, Inc., Philipsburg, NJ). Separation of acetylated and unacetylated forms was performed in a chloroform/methanol (95/5) ascending mobile phase followed by autoradiography at room temperature using a phosphor screen (Molecular Dynamics, Sunnyvale, CA). The radioactivity of each spot was quantitated with a PhosphorImager (Molecular Dynamics). All cell lines were transfected and analyzed on the same day for each independent experiment. The relative CAT activity in each lysate was quantitated by the following equation: ((Ac/(Ac +Uc)/(Ab/(Ab + Ub)), where A and U refer to the volumes of the acetylated and unacetylated forms of chloramphenicol, respectively, and c and b refer to CAT constructs and the pBasic control plasmid, respectively. The data were normalized for transfection efficiency based on relative β-galactosidase activity, which varied <10% among samples in each experiment.
Results
Identification of the 5′-UTR of CCR5 mRNA
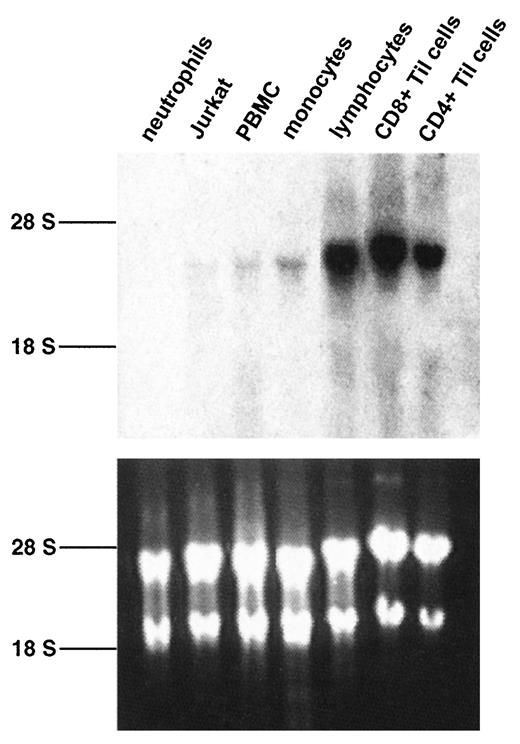
The promoters of all known chemoattractant receptor genes are separated from the ORF by one or more large introns (6). Therefore, to locate the CCR5 promoter, it was important to first establish the complete sequence of the 5′-UTR. To do this, we attempted to identify a rich natural source of CCR5 mRNA for primer extension analysis. A single 3.5-kb band was detected by Northern blot hybridization analysis using a CCR5 ORF probe in RNA from elutriated monocytes, freshly isolated PBMCs, nonadherent mononuclear cells, cultured CD4+ and CD8+ Til cells, and Jurkat cells, but not in neutrophils (Fig. 1). The level of CCR5 RNA was highest in the cultured Til cell samples, and CD4+ Til cell RNA was chosen for primer extension analysis (Fig. 2). To minimize the probability of cross-hybridization to related sequences, the CCR5 primer was chosen to correspond to a region that is highly divergent in even closely related chemokine receptors. In particular, the corresponding sequence of CCR2, the closest homologue of CCR5, has no significant sequence similarity to this primer sequence. Also, no significant matches were found with any sequence in the GenBank or EST databases. Two adjacent bands of equal intensity were identified that corresponded to products extending the CCR5 primer by 77 and 78 nucleotides, respectively. Thus, the CCR5 gene appears to have two alternative tsps. After subtracting the distance of the primer from the first codon, the length of the 5′-UTR of the longer of the two mRNAs was 54 nucleotides. This result corresponds exactly to the length of the 5′-UTR of the clone 134 CCR5 cDNA sequence reported by Raport et al. (30).
Expression of CCR5 mRNA in hemopoietic cells. Total RNA (10 μg/lane) from neutrophils, PBMC, elutriated monocytes (monocytes), nonadherent PBMCs (lymphocytes), Jurkat cells, and CD8+ and CD4+ Til cells was hybridized with a radiolabeled full-length CCR5 ORF probe under high stringency conditions. Top panel, Autoradiography of the hybridized blot using an intensifying screen for 14 days. Bottom panel, Picture of the corresponding agarose gel stained with ethidium bromide. The position of the ribosomal RNA bands is indicated on the left.
Expression of CCR5 mRNA in hemopoietic cells. Total RNA (10 μg/lane) from neutrophils, PBMC, elutriated monocytes (monocytes), nonadherent PBMCs (lymphocytes), Jurkat cells, and CD8+ and CD4+ Til cells was hybridized with a radiolabeled full-length CCR5 ORF probe under high stringency conditions. Top panel, Autoradiography of the hybridized blot using an intensifying screen for 14 days. Bottom panel, Picture of the corresponding agarose gel stained with ethidium bromide. The position of the ribosomal RNA bands is indicated on the left.
Identification of the CCR5 transcriptional start point by primer extension. A labeled primer specific for the CCR5 ORF was incubated in the presence and the absence of CD4+ Til cell poly(A)+ RNA and extended by reverse transcriptase. A control primer extension reaction in the presence and the absence of a defined mRNA was conducted in parallel using components provided in a kit (control RNA). Extended products were separated on a 6% acrylamide gel adjacent to a control sequencing reaction for determination of product length. The gel was dried and exposed to Kodak XAR film at −80°C for 5 days. The arrow indicates the longest extended product for CCR5 RNA, and the length is given from the first nucleotide of the corresponding initiating ATG codon.
Identification of the CCR5 transcriptional start point by primer extension. A labeled primer specific for the CCR5 ORF was incubated in the presence and the absence of CD4+ Til cell poly(A)+ RNA and extended by reverse transcriptase. A control primer extension reaction in the presence and the absence of a defined mRNA was conducted in parallel using components provided in a kit (control RNA). Extended products were separated on a 6% acrylamide gel adjacent to a control sequencing reaction for determination of product length. The gel was dried and exposed to Kodak XAR film at −80°C for 5 days. The arrow indicates the longest extended product for CCR5 RNA, and the length is given from the first nucleotide of the corresponding initiating ATG codon.
Structural organization of the CCR5 gene
We next used the 5′-UTR sequence as a probe to locate the CCR5 promoter on a human genomic clone. Six genomic clones were first isolated by screening a library with an ORF probe, and three of these hybridized with two oligonucleotide probes corresponding to nucleotides 1 to 21 and 18 to 42 of the full-length mRNA sequence. One of these, designated clone 6-2a, was chosen for further analysis. A 2.7-kb XbaI/EcoRI fragment of clone 6-2a hybridized to both 5′-UTR oligonucleotide probes and was therefore subcloned and sequenced. From the 5′ end, this fragment contained 1006 bp that did not match the CCR5 cDNA sequence, followed by the first 43 bp of the 5′-UTR sequence of the clone 134 CCR5 cDNA, followed by 1676 bp that did not match CCR5 cDNA sequence. The 5′ end of a 2.7-kb HindIII fragment of genomic clone 6-2a overlapped the 3′ end of the XbaI/EcoRI fragment by 70 bp. From the 5′ end, this fragment contained 292 bp that did not match CCR5 cDNA sequence, followed by the 3′-most 11 bp of the 5′-UTR, the complete ORF, and the 3′-UTR of the CCR5 cDNA sequence. The sequence interrupting the 5′-UTR sequence begins with the 5′ dinucleotide gt and ends with the 3′ dinucleotide ag, consistent with splice donor and acceptor sites. Thus, the CCR5 gene has two exons: the first containing 43 bp of the 5′-UTR, and the second containing 11 bp of the 5′-UTR and the entire ORF, interrupted by an 1898-bp intron (Fig. 3, A and B). A consensus TATA sequence is located 31 bp upstream of the tsp (Fig. 3 A).
DNA sequence and organization of the CCR5 gene. A, CCR5 gene sequence. The exon sequence is in capital letters, whereas the intron and the putative promoter region are in lower case. The first nucleotide of exon 1 was chosen as nucleotide +1. The putative TATA box as well as several consensus transcription factor binding sites are indicated. The last three nucleotides are the first codon of the CCR5 ORF. XbaI, EcoRI, and HindIII restriction sites are also indicated. An Alu repeat in the intron is underlined. B, Genomic organization of CCR5. Shaded box, ORF; unshaded large box, transcribed but untranslated sequence; unshaded small box, Alu repeat; X, XbaI; H, HindIII; E, EcoRI. Note that the 3′ limit of exon 2 has not been defined. A length standard is indicated at the upper right. The sequence has been deposited in GenBank (accession number AF032132).
DNA sequence and organization of the CCR5 gene. A, CCR5 gene sequence. The exon sequence is in capital letters, whereas the intron and the putative promoter region are in lower case. The first nucleotide of exon 1 was chosen as nucleotide +1. The putative TATA box as well as several consensus transcription factor binding sites are indicated. The last three nucleotides are the first codon of the CCR5 ORF. XbaI, EcoRI, and HindIII restriction sites are also indicated. An Alu repeat in the intron is underlined. B, Genomic organization of CCR5. Shaded box, ORF; unshaded large box, transcribed but untranslated sequence; unshaded small box, Alu repeat; X, XbaI; H, HindIII; E, EcoRI. Note that the 3′ limit of exon 2 has not been defined. A length standard is indicated at the upper right. The sequence has been deposited in GenBank (accession number AF032132).
Identification of the CCR5 promoter
This structural analysis strongly suggested that the gene region upstream of exon 1 would contain a functional promoter for CCR5. To test this, we constructed a series of chimeric reporter genes in which portions of the 1006-bp fragment upstream from exon 1 were subcloned upstream from the CAT ORF in the plasmid pCAT-basic. We measured CAT activity in the human lymphoid cell line Jurkat, the human myeloid cell line U937, and the human embryonic kidney cell line 293 transiently transfected with each of these constructs.
The longest CCR5 gene region tested (construct 1) contained 971 bp upstream from exon 1 cloned in the sense orientation upstream from CAT. This region stimulated reporter gene activity 25- and 80-fold over that observed with the pCAT-basic vector in Jurkat and U937 cells, respectively. In contrast, the same gene region tested in the reverse orientation relative to CAT (construct 2) lacked activity in both cell lines (Fig. 4). Together, these results indicate that this 971-bp gene region contains a functional promoter element for this system.
Deletion analysis of the CCR5 promoter. A, Construct map and data for relative CAT expression. The model for the region upstream from exon 1 is shown at the upper left. The length and orientation of each gene region used for chimeric CAT constructs are indicated by arrows, with the first and the last nucleotide enumerated at each end. The constructs are numbered 1 through 10 (C1 to C10 as indicated on the y-axis of the graph). The activity of the positive control pSV40 is indicated at the top of each graph. The CAT activity of each construct and that of pSV40 relative to the negative control pCAT-basic are the mean ± SEM based on the number of experiments indicated in the upper right of each panel. The cell type used for transfection is also indicated at the upper right of each panel. Data were corrected for differences in transfection efficiency, which were <10% among all samples in each experiment. Data for Jurkat and U937 cells are from electroporated cells; data for HEK 293 cells are from cells transfected by lipofection. The transfection efficiency of HEK 293 cells using electroporation was very low. B, Representative data for one experiment in Jurkat cells.
Deletion analysis of the CCR5 promoter. A, Construct map and data for relative CAT expression. The model for the region upstream from exon 1 is shown at the upper left. The length and orientation of each gene region used for chimeric CAT constructs are indicated by arrows, with the first and the last nucleotide enumerated at each end. The constructs are numbered 1 through 10 (C1 to C10 as indicated on the y-axis of the graph). The activity of the positive control pSV40 is indicated at the top of each graph. The CAT activity of each construct and that of pSV40 relative to the negative control pCAT-basic are the mean ± SEM based on the number of experiments indicated in the upper right of each panel. The cell type used for transfection is also indicated at the upper right of each panel. Data were corrected for differences in transfection efficiency, which were <10% among all samples in each experiment. Data for Jurkat and U937 cells are from electroporated cells; data for HEK 293 cells are from cells transfected by lipofection. The transfection efficiency of HEK 293 cells using electroporation was very low. B, Representative data for one experiment in Jurkat cells.
To identify a minimal promoter, we tested a series of nested fragments in the sense orientation relative to CAT containing the same 3′ end as that in construct 1, but with variably truncated 5′ ends (constructs 3–6). Construct 6 was the shortest construct tested, only 80 bp in length. This construct retained high activity relative to construct 1, with 15- and 30-fold greater CAT activity relative to the pCAT-basic vector in Jurkat and U937 cells, respectively. Thus, this gene region contains a minimal promoter in this system (Fig. 4). Interestingly, construct 5 (−244 to −1 bp), which contained the 80-bp active region plus an additional 164 bp upstream, lacked activity in both Jurkat and U937 cells, operationally defining the presence of a silencer element in the 164-bp region in this system (Fig. 4). Construct 4 (486 bp) contained an additional 242 bp upstream from the silencer region, which restored high promoter activity, with 50- and 140-fold increases relative to the pCAT-basic vector in Jurkat and U937 cells, respectively (Fig. 4). Thus, the region from −486 to −244 appears to contain an enhancer element for this system that can silence the downstream silencer element. However, when the region from −486 to −244 was tested independently in Jurkat cells (construct 9, Fig. 4 A), a 40-fold enhancement of reporter gene activity was observed, similar to that obtained with the longest gene region tested in construct 1. Thus, the region from −486 to −244 contains elements that can function independently as a promoter in this system.
Construct 3 (nucleotides −729 to −1 relative to the tsp) contains an additional 243-bp 5′ of construct 4, but had activity similar to that of construct 4, suggesting that the unshared gene region from −729 to −486 does not contain additional functional elements affecting reporter gene expression. Consistent with this, when this region was tested independently (construct 8, Fig. 4,A), no stimulation of CAT activity was observed. Finally, when we tested independently the 5′-most 242-bp gene region upstream from construct 8, we again observed no stimulation of CAT activity (construct 7 in Fig. 4,A, nucleotides −971 to −729). Construct 10, which tests the entire gene region tested separately in constructs 7 to 9, had activity similar to that observed for the gene region from −486 to −244 tested separately in construct 9 (Fig. 4 A). Thus, the 5′-most 485 bp of the parental 971-bp gene region do not affect the constitutive promoter activity found in region −486 to −244 or that found in region −1 to −80.
The relative activity for all constructs was the same whether the DNA was delivered to Jurkat or U937 cells by electroporation (Fig. 4 A) or lipofection (data not shown). In contrast, when we tested the constructs in human embryonic kidney epithelial 293 cells (HEK 293 cells), a cell line lacking endogenous CCR5 mRNA and CCR5 receptors, different transfection methods gave different results. With electroporated HEK 293 cells, CAT-promoting activity significantly greater than that of pCAT-Basic was not observed for constructs C1 through C6. However, transfection efficiency was very low, much lower than that for electroporated Jurkat and U937 cells, raising the possibility of false negative results in this cell type (data not shown). We therefore repeated the experiments using lipofection, which allowed high transfection efficiency of the cells, as assessed by β-galactosidase activity. Significant promoter activity was then observed for constructs C1, C3, C4, and C6, but not for C2 or C5, compared with the pCAT-Basic plasmid control. This is the same pattern of activity as that observed for the same constructs tested in Jurkat and U937 cells.
Analysis of the CCR5 sequence
When the CCR5 sequence was compared with the GenBank database using the Blast algorithm, a complete Alu repeat was identified from +1303 to +1587 relative to the tsp, placing it toward the end of the intron (Fig. 3). In the 1006-bp region upstream from exon 1, only short stretches of limited sequence identity with other human genes, including the CXCR1 and CXCR2 promoters, were found. The CCR5 promoter region has several sequences similar to consensus sequences for the transcription factors activating protein-1, CCAAT-binding transcription factor/nuclear factor-1 (NF-1), NF-κB, NF-ATp, and IFN-stimulated response element binding protein (Fig. 3 A).
Discussion
In the present report we have established the structural organization of the gene for CCR5 and located its functional promoter. Like most, but not all, G protein-coupled receptor genes, the CCR5 ORF lacks introns. This is also the case for CXCR1, CXCR2, CCR1, and CCR3, as well as for receptor genes for the nonchemokine classical chemoattractants fMet-Leu-Phe and platelet-activating factor (33, 34, 35, 36, 37, 38, 39). Exceptions to this pattern are CXCR3 and CXCR4, each of which has a large intron in the region encoding the N-terminal segment before transmembrane domain 1 (40) (S. K. Ahuja and P. M. Murphy, unpublished observations); CCR2, which has an intron in the region encoding the C-terminal cytoplasmic tail that is used to make two receptors by alternative splicing (41); and the C5a receptor, which has a large intron within the first codon (42).
Like genes for other chemokine receptors (e.g., CXCR1, CXCR2, and CCR1) and nonchemokine chemoattractant receptors (e.g., the fMet-Leu-Phe receptor and the platelet-activating factor receptor) and unlike most other G protein-coupled receptor genes, the CCR5 gene has a large intron interrupting the 5′-UTR sequence, placing the promoter and transcription start point at a considerable genomic distance upstream from the translation initiation site. CXCR2, CCR1, and the fMet-Leu-Phe receptor genes all have two or more introns in the 5′-UTR, and alternative splicing gives rise to multiple mRNA species with the same ORF sequence but different 5′-UTR sequences (33, 36) (J.-L. Gao and P. M. Murphy, unpublished observations). This does not appear to be the case for CCR5; variations in length, but not in sequence, have been found in the 5′-UTR for the two cDNA sequences that have been reported (24, 30), and a single ∼3.5-kb band has been consistently identified by Northern blot analysis of CCR5-expressing cells (24, 30, 43).
Although it is clear that the chemoattractant receptor ORFs have descended from a common ancestor, a common origin for their promoters is not apparent from sequence comparisons. Thus, the boundaries of the replication unit for chemoattractant receptor genes is not known. This is true even for CXCR1 and CXCR2, whose genes are clustered on human chromosome 2q35, expressed in neutrophils, and encode proteins with 78% amino acid identity, but have dissimilar promoter sequences (33, 44). It will be interesting to test whether this holds true for CCR5 and CCR2, which are the most closely related known CC chemokine receptors (75% amino acid identity).
To define regions responsible for constitutive CCR5 expression, we tested the activity of a chimeric CAT reporter gene containing 971 bp upstream from exon 1 in various cultured cell lines. This region promoted a high level of constitutive CAT activity, but in the sense orientation only, in both the T cell line Jurkat and the promonocytic cell line U937. Consistent with this, we and others have shown that endogenous CCR5 is constitutively expressed in both primary CD4+ and CD8+ T cells and monocytes (24, 26, 27). Also, we have detected a low level of CCR5 mRNA in Jurkat cells by Northern blot analysis (Fig. 1). Although this is consistent with the promoter activity we have found, it is important to note that we do not have evidence for expression of CCR5 protein in these cells as assessed by staining with a polyclonal antiserum to CCR5 or by calcium flux responses to CCR5 agonists (data not shown). It is also important to note that we have not identified endogenous CCR5 mRNA in U937 or HEK 293 cell samples, although the sensitivity of the analysis was low (Northern blot analysis of 10 μg of total RNA). Additional tissue-specific control sequences may be located outside the gene regions we have tested that affect tissue-specific expression of endogenous CCR5.
When we analyzed a series of deletion mutants of the active 971-bp region upstream from exon 1, we identified an 80-bp region immediately upstream from exon 1 that retained high constitutive promoter activity in the CAT reporter gene system when tested in both Jurkat and U937 cells. Since this region is near the tsp, contains a TATA sequence, and has promoter activity in the CAT reporter gene system, it appears to contain a minimal promoter and may be responsible for constitutive expression of endogenous CCR5 in myeloid and lymphoid cell types.
However, we also identified a second region, from −244 to −486 bp relative to the tsp, that also contained high constitutive promoter activity in the CAT reporter gene system when expressed in Jurkat cells. In contrast, the upstream gene regions from −486 to −729 and from −729 to −971 lacked independent promoter activity and did not significantly affect the constitutive activity of the two downstream promoters. A strong suppressor element must reside between nucleotides −244 to −80, since no promoter activity was observed with a construct between −244 to −1, which contains the 80-bp region that acts as a minimal promoter when tested independently. The activity of this silencer is overcome by the upstream region from −244 to −486, restoring the activity to the level found in the region from −1 to −80 alone.
The transcription factors responsible for constitutive and regulated expression of leukocyte chemoattractant receptors have not yet been defined. The CCR5 promoter region contains several sites with >80% identity with the consensus sequences for elements that bind the transcription factors CCAAT-binding transcription factor/NF-1, activating protein-1, NF-κB, and NF-ATp. Also, three IFN-stimulated response elements were identified, suggesting a possible modulation of the promoter activity by IFN. CCR5 expression has been reported to be slowly induced by IL-2 treatment of primary T cells and down-regulated by CD28 activation of PBMCs. However, whether the mechanism involves transcriptional or post-transcriptional regulation, or both, has not yet been determined. To date, we have not observed changes in promoter activity in our reporter gene system in response to the cytokines IL-2 and IFN-γ (data not shown).
The present study provides a foundation for future studies aimed at identifying protein factors and DNA sequences specifically responsible for CCR5 transcription. In addition, the promoter sequence we have described can now be used to screen cohorts of individuals who have been highly exposed to HIV-1 yet remain uninfected, to test whether additional inactivating CCR5 mutations exist that could confer natural resistance to HIV-1. An analogous precedent for this exists for another chemokine receptor known as the Duffy Ag, which acts pathologically as an erythrocyte-specific cell entry factor for the malaria-causing protozoan Plasmodium vivax (45). An inactivating mutation in a GATA-1 site is present in the Duffy promoter of most Africans and is responsible for natural resistance to vivax malaria (46). Finally, the CCR5 promoter sequence is a potential target for gene therapy for HIV-1 through triplex DNA and gene-targeting strategies.
Acknowledgements
We thank J. Farber for generously providing the Til cell lines.
Footnotes
This work was supported in part by a grant from the Swiss National Science Foundation (to F.G.).
The sequence of CCR5 has been deposited in GenBank (accession number AF032132).
Abbreviations used in this paper: CCR, CC chemokine receptor; Til cells, tumor-infiltrating T lymphocytes; ORF, open reading frame; UTR, untranslated region; CAT, chloramphenicol acetyltransferase; tsp, transcription start point; CXCR, CXC chemokine receptor.