Abstract
Binding of IL-2 to its receptor activates several biochemical pathways, including JAK-STAT, Ras-mitogen-activated protein kinase, and phosphatidylinositol 3′-kinase (PI 3′-kinase) pathways. Recently, it has been shown that the SH2-containing phosphatase, SHP-2, becomes phosphorylated in response to IL-2 stimulation, associates with PI3′-kinase and Grb2, and can exert a positive regulatory role in IL-2 signaling. We now report the identification of a prominent 98-kDa protein (p98) found to be phosphorylated in response to IL-2 stimulation and coprecipitated with SHP-2, the p85 subunit of PI 3′-kinase and Grb2. Interestingly, whereas IL-4 is known to activate PI 3′-kinase, we did not observe any p98 phosphorylation in response to IL-4 stimulation. p98 can form a multipartite complex with all these proteins as immunodepleting with anti-p85 antiserum substantially reduced the amount of p98 immunoprecipitated by SHP-2 and Grb2; the converse was also true. Furthermore, phosphorylation of p98 did not occur in cells lacking JAK3, suggesting that it may be a JAK substrate. Finally, deglycosylation of p98 did not alter its migration, suggesting p98 is not a member of the recently described SHP substrate/signal-regulatory proteins family of transmembrane glycoproteins. Thus p98 is a prominent IL-2-dependent substrate that associates with multiple proteins involved in IL-2 signaling and may play an important role in coupling the different signal transduction pathways activated by IL-2.
Interleukin-2 (IL-2) is a key cytokine that modulates immune responses by acting on several cellular subsets including T, B, NK cells, and monocytes 1, 2 . Binding of IL-2 to its receptor induces rapid tyrosine phosphorylation and activation of Janus family protein-tyrosine kinases JAK1 and JAK33 3, 4 . The current paradigm is that JAKs phosphorylate the IL-2R, 5 allowing STAT binding to the receptor 6, 7, 8, 9, 10 . Recruited STATs are also phosphorylated, permitting reciprocal dimerization and translocation to the nucleus.
The phosphorylated receptor is also recognized by the adapter molecule Shc. Shc in turn becomes phosphorylated, allowing the recruitment of another adapter protein Grb2 and the guanosine nucleotide exchange factor SOS, which mediate the activation of the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway 11, 12 . IL-2 also activates phosphatidylinositol 3′-kinase (PI 3′-kinase) 13, 14 and this is also thought to contribute to MAPK activation 15 . Neither the mechanism by which PI 3′-kinase activation occurs nor the steps by which PI 3′-kinase activation leads to MAPK activation have at present been clearly defined.
IL-2 and IL-4 have been reported to induce the phosphorylation of cytosolic docking proteins such as insulin receptor substrate (IRS-1) and IRS-2 16 . With IL-4, it is thought that the phosphotyrosine binding domain of IRS-1 and IRS-2 binds the phosphorylated receptor at Tyr497 and PI 3′-kinase binds phosphorylated IRS-1 or IRS-2. It is still not clear whether IL-2-dependent activation of MAPK involves IRS molecules, although it has alternatively been suggested that PI 3′-kinase binds directly to the IL-2 receptor 17 .
For transmembrane receptor tyrosine kinases, it appears that yet another means of MAPK activation do exist. With the platelet-derived growth factor β receptor, it has been demonstrated that the SH2 containing phosphatase (SHP-2), can bind to the receptor and act as an adapter between the receptor itself and the Grb2-SOS complex 18, 19 . Recently, it has been shown that SHP-2 is also phosphorylated in response to IL-2 20, 21 and that IL-2 stimulation induces SHP-2 association with the p85 subunit of PI 3′-kinase and Grb2 22 . Notably, we also showed that SHP-2 is important in IL-2-dependent MAPK activation, because catalytically inactive SHP-2 blocked the IL-2 induced activation of MAPK. Moreover, SHP-2 expression also amplified STAT-dependent transcriptional activation. Thus, as with growth factor receptors, SHP-2 may be important in coupling the IL-2 receptor to MAPK activation. The mechanism by which this occurs, however, is unclear.
To better understand how SHP-2 positively regulates MAPK in IL-2 signaling, we searched for SHP-2-associated proteins that became phosphorylated in response to IL-2 stimulation. Here, we describe a substrate that was found to be tyrosine phosphorylated in response to IL-2 and was associated with SHP-2, PI 3′-kinase, and Grb2. Importantly, we demonstrate that p98 phosphorylation was dependent on the presence of the IL-2-activated Janus kinase, JAK3. Taken together, our results suggest that p98 may serve an important role as an adapter molecule, facilitating the activation of a variety of signaling pathways induced by IL-2.
Materials and Methods
Cytokines and Abs
Human IL-2 and IL-4 were provided by Dr. C. Reynolds (National Cancer Institute, Frederick, MD) IL-7, IL-10 and IL-12 were purchased from R&D System (Minneapolis, MN), IFN-α/β was purchased from Genzyme (Cambridge, MA). The following Abs were purchased: antiphosphotyrosine Ab (Py-plus Mixture) (Zymed, San Francisco, CA); anti-PI 3′-kinase (Upstate Biotechnology, Lake Placid, NY), anti-GRB2, and anti-SHP-2 (Santa Cruz Biotechnology, Santa Cruz, CA); and mouse monoclonal anti-PTP1D (SHP-2) (Transduction Laboratories, Lexington, KY). Endoglycosidase F/N-glycosydase F was purchased from Boehringer Mannheim (Indianapolis, IN).
Cell culture
Human PBMC from peripheral blood of healthy donors were prepared as previously described (97% CD3+) 23 . The human NK cell line NK3.3 was kindly provided by Dr. J. Kornbluth (Arkansas Cancer Research Center, Little Rock, AR) and cultured as described 23 . Before stimulation, both T cells and NK3.3 were washed in CO2-acidified RPMI 1640 and rested for 24 h in RPMI 1640 containing 1.5% BSA.
Immunoprecipitation, immunoblotting, and deglycosylation
After resting, cells were resuspended in 1 ml of medium (3 × 107 NK3.3 cells, 5 × 107 T cells), incubated at 37°C and stimulated with IL-2 (1000 IU/ml), IL-4 (1000 IU/ml), IFN-α/β (1000 IU/ml), IL-7 (10 ng/ml), IL-10 (10 ng/ml), or IL-12 (10 ng/ml) for the indicated times. After stimulation, cells were washed once in PBS and lysed in buffer containing 0.5% Triton X-100, 50 mM Tris-HCl (pH 7.5), 300 mM NaCl, 2 mM EDTA, 200 μM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 2.5 μM p-nitrophenyl p-guanidinobenzoate on ice for 30 min. Immunoprecipitation, PAGE, and immunoblotting were performed as described previously 3 . Proteins were detected by enhanced chemiluminescence (LumiGLO, Kirkegaard & Perry Laboratories, Gaithersburg, MD). Deglycosylation experiments were performed according to Kharitonenkov et al. 24 . Briefly, immunoprecipitates were boiled for 5 min in buffer containing 1% SDS. They were then suspended in 500 μl of deglycosylation buffer (40 mM potassium phosphate (pH 7.0), 20 mM EDTA, 1% 2-ME, 1% Triton X-100) with 0.5 U of endoglycosidase F/N-glycosidase F and incubated at 37°C for 18 h. PAGE and immunoblotting were performed as described above.
Results
Stimulation with IL-2 results in the appearance of SHP-2-associated phosphoproteins in T and NK cells
As previously reported, SHP-2 is phosphorylated in response to IL-2 stimulation 20, 21 . Interestingly, SHP-2 itself was not the only phosphoprotein found in SHP-2 immunoprecipitates from freshly isolated T cells. In addition to phosphorylation of SHP-2, we also noted a prominent phosphorylated substrate with an apparent molecular mass of ∼98 kDa that was coimmunoprecipitated with SHP-2 (Fig. 1,A, lane 2). We also noticed the presence of a band at ∼130 kDa that was weakly constitutively phosphorylated in resting T cells and was further phosphorylated after IL-2 stimulation. Notably, other cytokines, including those that also activate JAK3 (IL-4 and IL-7), did not induce SHP-2 phosphorylation. Furthermore, the cytokines that failed to induce SHP-2 phosphorylation also did not induce p98 phosphorylation. To confirm that equal amounts of SHP-2 were immunoprecipitated in the different samples, the filter was reblotted with anti-SHP-2 (Fig. 1,A, lower panel). The specificity of the immunoprecipitated bands was verified by immunoprecipitating with a nonimmune control serum (Fig. 1 A, lanes 7 and 8).
IL-2 selectively induces the appearance of SHP-2-associated phosphoproteins in T and NK cells. A, T cells, untreated (lanes 1 and 7), or treated for 5 min with IL-2 (lanes 2 and 8), IL-4 (lane 3), IL-7 (lane 4), IL-12 (lane 5), or IFN-αβ (lane 6), were lysed and immunoprecipitated with anti-SHP-2 antiserum and then subjected to immunoblotting with antiphosphotyrosine (top) and anti-SHP-2 (bottom). B, NK3.3 cells, untreated (lane 1) or treated with IL-2 (lane 2), IL-12 (lane 3), IL-4 (lane 4), or IFN-αβ (lane 5) for 5 min were lysed and immunoprecipitated with anti-SHP-2 antiserum and then subjected to immunoblotting with antiphosphotyrosine (top) and anti-SHP-2 (bottom). C, NK3.3 cells, untreated (lane 1) or treated with IL-2 for 5 min (lane 2), 10 min (lane 3), 15 min (lane 4), or 30 min (lane 5) were lysed and immunoprecipitated with anti-SHP-2 antiserum and then subjected to immunoblotting with antiphosphotyrosine (top) and anti-SHP-2 (bottom).
IL-2 selectively induces the appearance of SHP-2-associated phosphoproteins in T and NK cells. A, T cells, untreated (lanes 1 and 7), or treated for 5 min with IL-2 (lanes 2 and 8), IL-4 (lane 3), IL-7 (lane 4), IL-12 (lane 5), or IFN-αβ (lane 6), were lysed and immunoprecipitated with anti-SHP-2 antiserum and then subjected to immunoblotting with antiphosphotyrosine (top) and anti-SHP-2 (bottom). B, NK3.3 cells, untreated (lane 1) or treated with IL-2 (lane 2), IL-12 (lane 3), IL-4 (lane 4), or IFN-αβ (lane 5) for 5 min were lysed and immunoprecipitated with anti-SHP-2 antiserum and then subjected to immunoblotting with antiphosphotyrosine (top) and anti-SHP-2 (bottom). C, NK3.3 cells, untreated (lane 1) or treated with IL-2 for 5 min (lane 2), 10 min (lane 3), 15 min (lane 4), or 30 min (lane 5) were lysed and immunoprecipitated with anti-SHP-2 antiserum and then subjected to immunoblotting with antiphosphotyrosine (top) and anti-SHP-2 (bottom).
To determine whether this 98-kDa substrate was present in other IL-2 responsive cells we repeated the experiments using the NK3.3 cell line. As shown in Fig. 1,B when cells were stimulated with IL-2 for 5 min (Fig. 1,B, lane 2), lysed and immunoprecipitated with anti-SHP-2, phosphorylation of p98 was again evident, although in this particular cell line SHP-2 phosphorylation was minimal compared with normal T cells. In contrast, stimulation of cells with IL-12, IL-4, or IFN-α/β did not induce SHP-2 or p98 phosphorylation (Fig. 1,B, lanes 3–5) despite the responsiveness of the cells to these cytokines as measured by phosphorylation of molecules such as STAT4 (for IL-12 and IFN-α/β) or STAT6 (for IL-4) (data not shown). A time-course experiment (Fig. 1 C) showed that the tyrosine phosphorylation of p98 was evident as early as 5 min of stimulation, peaked between 5 and 10 min and declined after 30 min of IL-2 treatment.
It should be noted that because p98 was detected in SHP-2 immunoprecipitates, we cannot distinguish at present whether p98 itself is inducibly or constitutively phosphorylated or whether its association with SHP-2 is regulated by IL-2 stimulation; such experiments will require specific p98 reagents. However, because SHP-2 has SH2 domains, it is plausible to speculate that the association between SHP-2 and p98 might be phosphotyrosine dependent. Thus p98 might serve as a phosphorylated docking protein similar to IRS proteins. We therefore sought to analyze this phosphoprotein in greater detail.
IL-2 induces the association of p98 with PI 3′-kinase
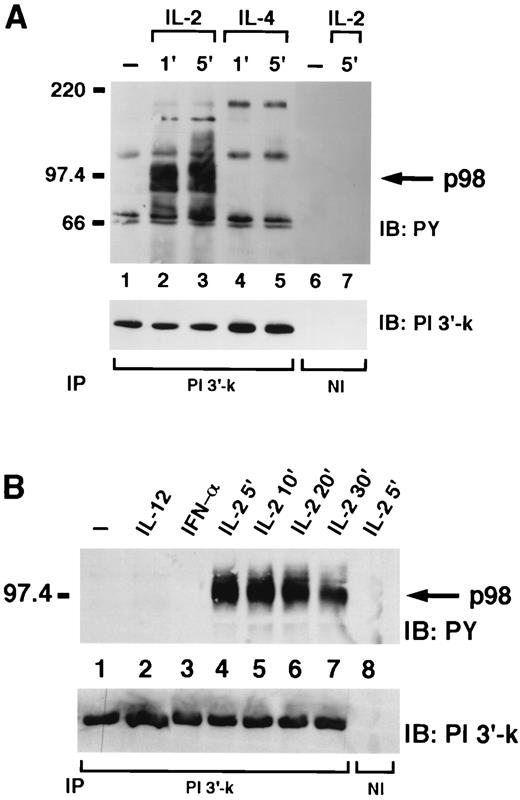
In our previous study, we observed that IL-2 induced association of SHP-2 with PI 3′-kinase. We speculated that one way this might occur would be if SHP-2 and PI 3′-kinase both bound p98. Therefore, we next investigated whether a 98-kDa phosphoprotein could also be detected in association with the p85 subunit of PI 3′-kinase from IL-2-stimulated cells. To this end, NK3.3 cells were stimulated with IL-2 or IL-4 and immunoprecipitated with anti-p85 antiserum. As shown in Fig. 2,A, we observed a number of phosphorylated proteins that coimmunoprecipitated with PI 3′-kinase. Importantly, a band at ∼98 kDa was present in IL-2 stimulated cells but not in IL-4 stimulated cells. The IL-4 treatment, however, was able to induce the phosphorylation of a molecule with apparent molecular mass of 180 kDa, which was presumably IRS-1 (lanes 4 and 5) indicating that IL-4 did induce substrate phosphorylation in these cells. Equivalent amount of loading was confirmed by reprobing the filter with anti-p85 antiserum (Fig. 2 A, lower panel).
IL-2 selectively induces the appearance of a 98-kDa phosphoprotein associated with the p85 subunit of PI 3′-kinase. A, NK3.3 cells, untreated (lanes 1 and 6) or treated with IL-2 or IL-4 for 1 min (lanes 2 and 4) or 5 min (lanes 3, 5, and 7), were lysed and immunoprecipitated with anti-p85 (lanes 1–5) or nonimmune antiserum (lane 6–7) and then subjected to immunoblotting with antiphosphotyrosine (top) or anti-p85 antiserum (bottom). B, NK3.3 cells, untreated (lane 1) or treated with IL-12 (lane 2), IFN-α/β (lane 3), IL-2 for 5 min (lanes 4 and 8) or IL-2 for 10 min (lane 5), IL-2 for 20 min (lane 6), IL-2 for 30 min (lane 7), were lysed and immunoprecipitated with anti-p85 antiserum (lanes 1–7) or nonimmune antiserum (lane 8) and then subjected to immunoblotting with antiphosphotyrosine (top) or anti-p85 antiserum (bottom).
IL-2 selectively induces the appearance of a 98-kDa phosphoprotein associated with the p85 subunit of PI 3′-kinase. A, NK3.3 cells, untreated (lanes 1 and 6) or treated with IL-2 or IL-4 for 1 min (lanes 2 and 4) or 5 min (lanes 3, 5, and 7), were lysed and immunoprecipitated with anti-p85 (lanes 1–5) or nonimmune antiserum (lane 6–7) and then subjected to immunoblotting with antiphosphotyrosine (top) or anti-p85 antiserum (bottom). B, NK3.3 cells, untreated (lane 1) or treated with IL-12 (lane 2), IFN-α/β (lane 3), IL-2 for 5 min (lanes 4 and 8) or IL-2 for 10 min (lane 5), IL-2 for 20 min (lane 6), IL-2 for 30 min (lane 7), were lysed and immunoprecipitated with anti-p85 antiserum (lanes 1–7) or nonimmune antiserum (lane 8) and then subjected to immunoblotting with antiphosphotyrosine (top) or anti-p85 antiserum (bottom).
As further evidence that the SHP-2 and PI 3′-kinase p98 substrates were related, we again analyzed the effect of stimulation with different cytokines. As previously shown for SHP-2, only IL-2 was able to induce phosphorylation of the PI 3′-kinase-associated p98. Moreover, phosphorylation of the PI 3′-kinase-associated p98 was maximal between 5 and 10 min and decreased by 30 min (Fig. 2,B, lanes 4–7), identical with the time course of phosphorylation of the SHP-2-associated p98. The difference in levels of p98 phosphorylation was not due to different amounts of immunoprecipitated PI 3′-kinase because reprobing of the same filter with p85 antiserum demonstrated equal amount of loading in every lane (Fig. 2,B, lower panel). Specificity was ascertained again by immunoprecipitating an IL-2-stimulated cells lysate with nonimmune serum (Fig. 2 B, lane 8).
IL-2 induces the association of p98 with Grb2
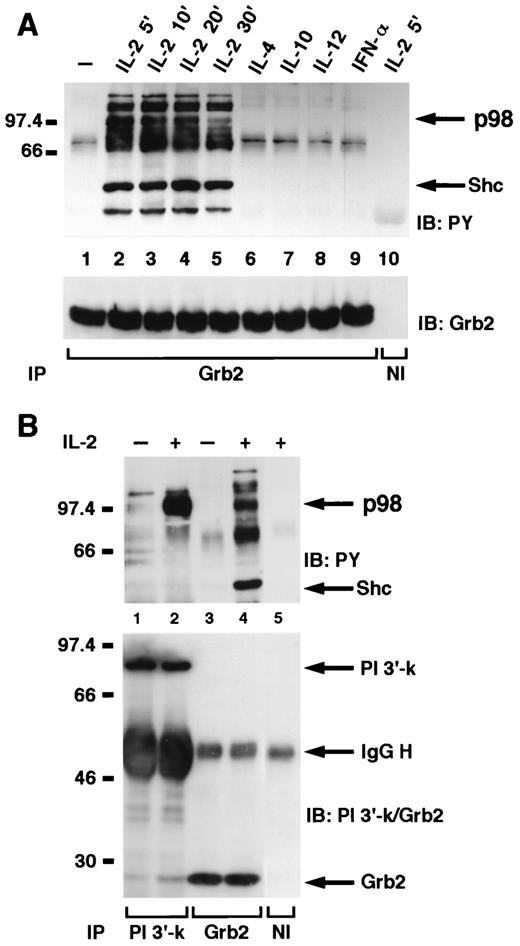
These results were consistent with a model in which p98 might serve as an adapter/docking molecule in IL-2 signaling, analogous to the role of IRS-1 or IRS-2 in IL-4 such that SHP-2 and PI 3′-kinase both bind p98 upon IL-2 stimulation. Because we had previously demonstrated 16 that SHP-2 was also associated with Grb2 in IL-2-stimulated cells, we next investigated whether p98 could also be observed in Grb2 immunoprecipitates after IL-2 stimulation. Several phosphophorylated bands were observed in lysates of NK3.3 cells after IL-2 stimulation (Fig. 3,A), the adapter molecule Shc being readily recognizable (lower arrow). In addition, as already noted in SHP-2 and PI 3′ kinase immunoprecipitates from IL-2 stimulated cells, we detected a broad tyrosine-phosphorylated band that migrated at ∼98 kDa, phosphorylation of which was maximal at ∼5 min and greatly diminished by 30 min. The phosphorylation of this 98 kDa was not observed when cells were stimulated with either IL-4, IL-10, IL-12, or IFN-α/β, despite the fact that Grb2 was equally immunoprecipitated from these cells (Fig. 3,A, lower panel). To better compare the pattern of IL-2-induced phosphoproteins associated with Grb2 and PI 3′-kinase, we also used a 7–12.5% polyacrylamide gradient gel that allowed a better separation of proteins. As shown in Fig. 3 B, we found a similar 98-kDa phosphoprotein associated with both PI 3′-kinase and Grb2.
p98 is present in Grb2 immunoprecipitates following IL-2 stimulation and comigrates with the PI 3′-kinase associated substrate.A, NK3.3 cells, untreated (lane 1) or treated with IL-2 for 5 min (lanes 2 and 10), 10 min (lane 3), 20 min (lane 4), or 30 min (lane 5) or treated with IL-4 (lane 6), IL-10 (lane 7), IL-12 (lane 8), or IFN-α/β (lane 9), for 5 min were lysed, immunoprecipitated with anti-Grb2 antiserum (lanes 1–9) or nonimmune antiserum (lane 10), and then subjected to PAGE on a 10% gel, following immunoblotting with antiphosphotyrosine (top) and anti-Grb2 (bottom). B, NK3.3 cells, untreated (lanes 1 and 3) or treated with IL-2 for 5 min (lanes 2, 4, and 5), were lysed and immunoprecipitated with anti-p85 antiserum (lanes 1 and 2), anti-Grb2 antiserum (lanes 3 and 4), or nonimmune antiserum (lane 5) and then subjected to PAGE on a 7–12.5% gradient gel immunoblotting with antiphosphotyrosine (top) and anti-p85 and Grb2 (bottom).
p98 is present in Grb2 immunoprecipitates following IL-2 stimulation and comigrates with the PI 3′-kinase associated substrate.A, NK3.3 cells, untreated (lane 1) or treated with IL-2 for 5 min (lanes 2 and 10), 10 min (lane 3), 20 min (lane 4), or 30 min (lane 5) or treated with IL-4 (lane 6), IL-10 (lane 7), IL-12 (lane 8), or IFN-α/β (lane 9), for 5 min were lysed, immunoprecipitated with anti-Grb2 antiserum (lanes 1–9) or nonimmune antiserum (lane 10), and then subjected to PAGE on a 10% gel, following immunoblotting with antiphosphotyrosine (top) and anti-Grb2 (bottom). B, NK3.3 cells, untreated (lanes 1 and 3) or treated with IL-2 for 5 min (lanes 2, 4, and 5), were lysed and immunoprecipitated with anti-p85 antiserum (lanes 1 and 2), anti-Grb2 antiserum (lanes 3 and 4), or nonimmune antiserum (lane 5) and then subjected to PAGE on a 7–12.5% gradient gel immunoblotting with antiphosphotyrosine (top) and anti-p85 and Grb2 (bottom).
p98 forms a complex that comprises both SHP-2 and the p85 subunit of PI 3′-kinase
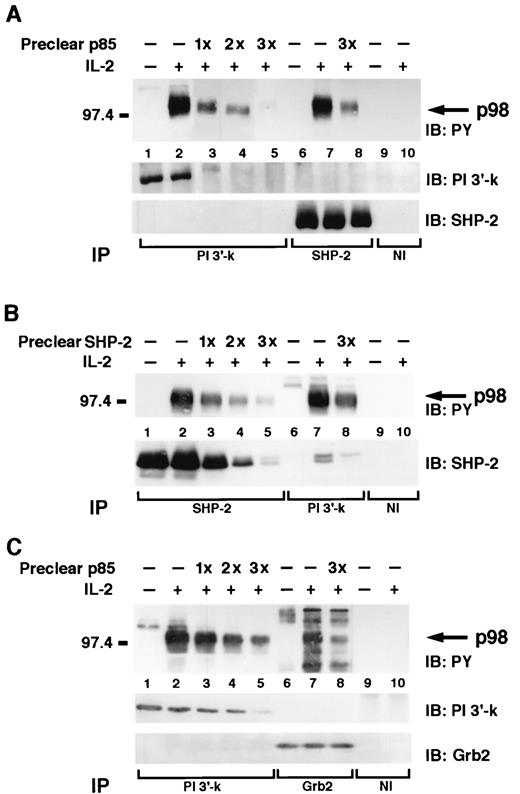
Thus our data indicated that SHP-2, PI 3′-kinase, and Grb2 all coprecipitated a 98-kDa phosphoprotein. Although it was phosphorylated by the same restricted stimulus (IL-2, but not other cytokines) with the same kinetics and had a similar migration in polyacrylamide gels, the SHP-2, PI 3′-kinase, and Grb2-associated p98 polypeptides might nonetheless have had no relationship to one another. To better define the nature of the p98 phosphoprotein found in the immunoprecipitates, we next performed sequential immunodepletion experiments. That is, IL-2 stimulated cells were lysed and extensively depleted with PI 3′-kinase antiserum or SHP-2 antiserum followed by immunoprecipitation with either PI 3′-kinase and SHP-2 (Fig. 4,A); SHP-2 and PI 3′-kinase (Fig. 4,B); or PI 3′-kinase and Grb-2 (Fig. 4,C). We found that the SHP-2-associated p98 as well as the Grb2-associated p98 were greatly diminished after p85 immunodepletion (Figs. 4,A, lane 8 and 4C, lane 8, respectively). Similarly, when lysates were immunoprecipitated with SHP-2 antiserum the PI 3′-kinase-associated p98 was also greatly reduced (Fig. 4 B, lane 8). These data strongly suggest that SHP-2, PI 3′-kinase, and Grb-2 are indeed associated with the same 98-kDa phosphoprotein and apparently form a multipartite complex; i.e., IL-2 stimulation results in the formation of a single complex comprising all these molecules. However, because some residual p98 was still detected in the different immunoprecipitates despite the extensive preclearing, p98 may also participate in distinct complexes with SHP-2, PI 3′-kinase, and Grb2 individually.
p98 participates in a complex that comprises PI 3′-kinase, SHP-2, and Grb2. A, NK3.3, untreated (lanes 1, 6, and 9) or treated with IL-2 for 5 min (lanes 2–5, 7, 8, and 10) were lysed and immunodepleted with p85 antiserum (preclear p85) (A) with SHP-2 antiserum (preclear SHP-2; B) and with p85 antiserum (preclear p85; C) (lanes 3–5 and 8, respectively), subjected to immunoprecipitation with the indicated Abs, and then subjected to immunoblotting analysis with the indicated Abs.
p98 participates in a complex that comprises PI 3′-kinase, SHP-2, and Grb2. A, NK3.3, untreated (lanes 1, 6, and 9) or treated with IL-2 for 5 min (lanes 2–5, 7, 8, and 10) were lysed and immunodepleted with p85 antiserum (preclear p85) (A) with SHP-2 antiserum (preclear SHP-2; B) and with p85 antiserum (preclear p85; C) (lanes 3–5 and 8, respectively), subjected to immunoprecipitation with the indicated Abs, and then subjected to immunoblotting analysis with the indicated Abs.
p98 is not a SHP substrate/signal-regulatory proteins (SHPS/SIRP) family member
Recently, a new class of transmembrane glycoproteins named SHPS or SIRPs that associate with SHP-1 and SHP-2 has been identified 24, 25 . Moreover, SIRP1-α has been shown to be phosphorylated in response to growth hormone, a cytokine that is related in a number of respect to IL-2. To clarify whether p98 was a member of this family, we performed an in vitro deglycosylation of SHP-2 and PI 3′-kinase immunoprecipitates because the mobility of SIRP1-α is greatly altered by this treatment 24, 26 . However, as shown in Fig. 5, deglycosylation did not affect the mobility of p98 in either SHP-2 or PI 3′-kinase immunoprecipitates. In contrast, enzymatic treatment of immunoprecipitated IL-2R β-chain, a known glycosylated transmembrane protein, clearly altered its migration, indicating that the treatment was indeed effective. These results indicate that p98 is not a glycosylated molecule and therefore not likely to be a member of the SHPS/SIRP family. Moreover, we were unable to detect any p98 or SHP-2 association with the IL-2R β-chain, suggesting that the p98 complex may not directly bind to the IL-2R.
p98 is not a glycosylated molecule. NK3.3 cells untreated (lanes 2, 5, 7, and 9) or treated for 5 min with IL-2 (lanes 1, 3, 4, 6, 8, and 10) were lysed, immunoprecipitated with the indicated Abs Immunoprecipitates were incubated with (lanes 2, 3, 5, 6, 9, and 10) or without (lanes 1, 4, 7, and 8) endoglycosidase F/N-glycosidase F and subsequently subjected to PAGE and immunoblotting with antiphosphotyrosine.
p98 is not a glycosylated molecule. NK3.3 cells untreated (lanes 2, 5, 7, and 9) or treated for 5 min with IL-2 (lanes 1, 3, 4, 6, 8, and 10) were lysed, immunoprecipitated with the indicated Abs Immunoprecipitates were incubated with (lanes 2, 3, 5, 6, 9, and 10) or without (lanes 1, 4, 7, and 8) endoglycosidase F/N-glycosidase F and subsequently subjected to PAGE and immunoblotting with antiphosphotyrosine.
p98 phosphorylation is JAK3 dependent
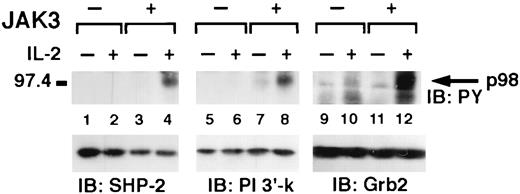
Because we have previously reported that JAK3 is important for IL-2-induced association of SHP-2 with Grb2 and PI 3′-kinase 22 , we next investigated whether JAK3 was required for the IL-2-induced phosphorylation of p98. Cells from patients with JAK3-SCID were first used to address this issue, but EBV-transformed B cells had constitutive phosphorylation of various substrates in the same molecular mass range of p98; this high level of basal phosphorylation excluded the use these cells to test the requirement of Jak3 in IL-2-induced p98 phosphorylation. We therefore used NIH 3T3 fibroblasts stably transfected with the IL-2R subunits with or without JAK3 (NIH 3T3 αβγ and NIH 3T3 αβγ-JAK3) 5, 27 . In the absence of JAK3, no phosphorylation of p98 was observed after IL-2 stimulation in NIH 3T3 αβγ cells (Fig. 6, lane 2) but was evident in SHP-2 immunoprecipitates from NIH 3T3 αβγ-JAK3 cells (lane 4). The same JAK3-dependent phosphorylation of p98 was observed in PI 3′-kinase (Fig. 6, lanes 5–8) and Grb2 (lanes 9–12) immunoprecipitates, although for the latter there was a higher phosphorylation in the absence of stimulation. We interpret these data to show that IL-2-dependent p98 phosphorylation may be Jak3 dependent, however we recognize that IL-2 receptor transfected fibroblasts are an imperfect model system to test this issue. Clearly a Jak3-deficient lymphoid line would be preferable although, as mentioned, we were unable to use EBV-transformed B cell lines from Jak3-deficient patients. The presence of SHP-2, PI 3′-kinase, Grb2-associated phosphoprotein in fibroblasts also suggests that p98 may be a ubiquitous and not lineage specific, protein. However, we have no direct evidence that the p98 found in murine fibroblasts is related to the polypeptide present in lymphocytes.
IL-2 mediated p98 phosphorylation is JAK3 dependent. NIH 3T3 αβγ (JAK3−) and NIH 3T3 αβγ-JAK3 (JAK3+) cells were untreated (lanes 1, 3, 5, 7, 9, and 11) or IL-2 treated for 5 min (lanes 2, 4, 6, 8, 10, and 12), immunoprecipitated with anti-SHP-2 (lanes 1-4) or anti-p85 (lane 5–8) or anti-Grb2 (lanes 9–12) and then subjected to PAGE and immunoblotting with antiphosphotyrosine (top panels). Filters were also reblotted with the indicated Abs (bottom panels).
IL-2 mediated p98 phosphorylation is JAK3 dependent. NIH 3T3 αβγ (JAK3−) and NIH 3T3 αβγ-JAK3 (JAK3+) cells were untreated (lanes 1, 3, 5, 7, 9, and 11) or IL-2 treated for 5 min (lanes 2, 4, 6, 8, 10, and 12), immunoprecipitated with anti-SHP-2 (lanes 1-4) or anti-p85 (lane 5–8) or anti-Grb2 (lanes 9–12) and then subjected to PAGE and immunoblotting with antiphosphotyrosine (top panels). Filters were also reblotted with the indicated Abs (bottom panels).
Discussion
IL-2 has been reported to induce the phosphorylation of the SH2 containing phosphatase SHP-2 20, 21 . Moreover, we have recently shown that SHP-2 associates with PI 3′-kinase and the adapter molecule Grb2, and by acting on the MAPK pathway, SHP-2 may exert a positive regulatory role in IL-2 signaling 22 . In this paper, we identified and characterized a 98-kDa protein that is phosphorylated in T cells and in NK cells in response to IL-2 but not to other cytokines including: IL-4, IL-7, IL-10, IL-12, or IFN-α/β. This polypeptide, referred to as p98, was coimmunoprecipitated with SHP-2, and was the most prominent phosphorylated band in SHP-2 immunoprecipitates. This is important, because characterization of p98 may help to clarify how IL-2 signaling is linked to the MAPK and other pathways.
We previously observed that IL-2 was able to induce the formation of SHP-2-PI 3′-kinase complex but we were not able to draw any conclusion on the nature of this complex. We therefore tested the possibility that the interaction could be mediated by a common intermediate. Interestingly, when we analyzed the phosphorylated substrates that were immunoprecipitated with the p85 subunit of PI 3′-kinase, we observed a major phosphorylated band that also comigrated with the SHP-2-associated phosphoprotein; indeed both polypeptides were phosphorylated with the same specificity and kinetics. More importantly, we found that preclearing with anti-PI 3′-kinase antiserum greatly diminished the SHP-2-associated p98, and the converse was also demonstrated to be true. Therefore, we hypothesize that the three molecules are part of a multiprotein complex. However, because we observed residual binding of p98 after extensive preclearing, the possibility also exists that p98 participates in complexes with each partner individually. This is in agreement with the fact that the consensus sequence for binding to the SH2 domains of SHP-2 and PI 3′-kinase p85 subunit are distinct. It is therefore tempting to speculate that SHP-2, p85, and possibly Grb2 bind to different phosphotyrosines on p98 and that p98 may function as an adapter protein.
We first considered that the SHP-2-associated p98 phosphoprotein seen with IL-2 stimulation might be a member of the recently described family of transmembrane glycoproteins with sequence homology to named SHPS/SIRPs 24, 25 , as SIRP1α is phosphorylated in response to growth hormone 26, 28 . However, the IL-2-dependent phosphoprotein p98 was not sensitive to deglycosylation treatment reducing the likelihood that it is a member of this family.
DOS (daughter of sevenless) is a substrate of the Drosophila homolog of SHP-2, corkscrew, and its mammalian homolog is Gab1 30, 31, 33 . We also considered the possibility that p98 is Gab1 but we were unable to detect Gab1 phosphorylation after IL-2 stimulation; indeed Gab1 was poorly expressed in the NK3.3 cell line (data not shown). Thus we interpret our results to indicate that p98 is unlikely to be Gab1 or a SIRP family member.
Recently, other groups have described SHP-2-associated and PI 3′-kinase-associated phosphoproteins that migrate at ∼100 kDa after cytokine stimulation 34, 35, 36, 37 . It is possible that the IL-2-activated p98 molecule we describe here is identical or related to these other proteins. It is notable that we found a p98-like molecule in IL-2-stimulated fibroblasts suggesting that p98 might be an ubiquitous protein that could play a role in signaling through a variety of receptors.
It is also of interest that IL-4 failed to induce p98 phosphorylation despite the commonalities in IL-2 and IL-4 signal transduction and the fact that IL-4 induces IRS phosphorylation. Given that the biological effects of IL-2 and IL-4 clearly differ, it is clearly important to identify distinct elements in their receptor signaling pathways.
Mitogenic signal in response to IL-2 has been demonstrated to be dependent on the presence of JAK3 41 . Although our results, obtained in a nonlymphoid system, suggest that, JAK3 may be required for p98 phosphorylation. The exact role of Jak3 in p98 phosphorylation will clearly need to be revisited when specific p98 reagents become available. In conclusion, although the functions of SHP-2 and its substrates are still poorly defined, if p98 acts as a docking molecule similar to the IRS it may play critical role in IL-2-induced T cell proliferation analogous to the role of IRS in IL-4-dependent proliferation. The identification and cloning of this molecule will be important to assess the significance of the SHP-2/PI 3′-kinase/Grb2/p98 complex and in defining the role of these molecules in IL-2 actions.
Note added in proof.
Since the submission of this manuscript Gesbert et al. 42 described a similar phosphorylated substrate.
Acknowledgements
We thank Dr. J. Kornbluth and Dr. T. Taniguchi for respectively providing NK 3.3 and NIH 3T3 αβγ cells. We are also indebted to Dr. Elbert H. Chen for helpful suggestions and critical comments.
Footnotes
Abbreviations used in this paper: JAK, Janus Kinase; MAPK, mitogen-activated protein kinase; IRS, insulin receptor substrate; PDGF, platelet-derived growth factor β; SHP-2, SH2-containing phosphatase; SHPS/SIRPs, SHP substrate/signal-regulatory proteins. PI 3′-kinase, phosphatidylinositol 3′-kinase.